Abstract
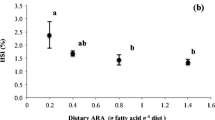
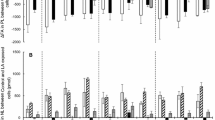
A two part experiment was conducted to assess the response of barramundi (Lates calcarifer; initial weight = 10.3 ± 0.03 g; mean ± S.D.) fed one of five diets with varying eicosapentaenoic acid (diets 1, 5, 10, 15 and 20 g/kg) or one of four diets with varying arachidonic acid (1, 6, 12, 18 g/kg) against a fish oil control diet. After 6 weeks of feeding, the addition of EPA or ARA did not impact on growth performance or feed utilisation. Analysis of the whole body fatty acids showed that these reflected those of the diets. The ARA retention demonstrated an inversely related curvilinear response to either EPA or ARA. The calculated marginal utilisation efficiencies of EPA and ARA were high (62.1 and 91.9 % respectively) and a dietary ARA requirement was defined (0.012 g/kg0.796/day). The partial cDNA sequences of genes regulating eicosanoid biosynthesis were identified in barramundi tissues, namely cyclooxygenase 1 (Lc COX1a, Lc COX1b), cyclooxygenase 2 (Lc COX2) and lipoxygenase (Lc ALOX-5). Both Lc COX2 and Lc ALOX-5 expression in the liver tissue were elevated in response to increasing dietary ARA, meanwhile expression levels of Lc COX2 and the mitochondrial fatty acid oxidation gene carnitine palmitoyltransferase 1 (Lc CPT1a) were elevated in the kidney. A low level of EPA increased the expression of Lc COX1b in the liver. Consideration should be given to the EPA to ARA balance for juvenile barramundi in light of nutritionally inducible nature of the cyclooxygenase and lipoxygenase enzymes.




Similar content being viewed by others
Abbreviations
- ARA:
-
Arachidonic acid (20:4n-6)
- C18PUFA:
-
Polyunsaturated fatty acid(s)
- cDNA:
-
Complementary deoxyribonucleic acid
- COX:
-
Cyclooxygenase (prostaglandin G/H synthase)
- DHA:
-
Docosahexaenoic acid (22:6n-3)
- EPA:
-
Eicosapentaenoic acid (20:5n-3)
- Lc:
-
Lates calcarifer
- LC-PUFA:
-
Long chain-polyunsaturated fatty acid(s)
- LOX:
-
Lipoxygenase (arachidonate 5-lipoxygenase)
- MUFA:
-
Monounsaturated fatty acid(s)
- RNA:
-
Ribonucleic acid
- SFA:
-
Saturated fatty acid(s)
References
Rowley AF, Knight J, Lloyd-Evans P, Holland JW, Vickers PJ (1995) Eicosanoids and their role in immune modulation in fish—a brief overview. Fish Shellfish Immunol 5:549–567
Wall R, Ross RP, Fitzgerald GF, Stanton C (2010) Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr Rev 68:280–289
Tocher DR (2003) Metabolism and functions of lipids and fatty acids in teleost fish. Rev Fish Sci 11:107–184
Calder PC (2012) Mechanisms of action of (n-3) fatty acids. J Nutr 142:592S–599S
Rouzer CA, Marnett LJ (2009) Cyclooxygenases: structural and functional insights. J Lipid Res 50(Suppl):S29–S34
Matsumoto T, Funk CD, Rådmark O, Höög JO, Jörnvall H, Samuelsson B (1988) Molecular cloning and amino acid sequence of human 5-lipoxygenase. Proc Natl Acad Sci 85:26–30
Ishikawa T, Herschman HR (2007) Two inducible, functional cyclooxygenase-2 genes are present in the rainbow trout genome. J Cell Biochem Suppl 102:1486–1492
Ishikawa T, Griffin KJP, Banerjee U, Herschman HR (2007) The zebrafish genome contains two inducible, functional cyclooxygenase-2 genes. Biochem Biophys Res Commun 352:181–187
Olsen RE, Svardal A, Eide T, Wargelius A (2012) Stress and expression of cyclooxygenases (cox1, cox2a, cox2b) and intestinal eicosanoids, in Atlantic salmon, Salmo salar L. Fish Physiol Biochem 38:951–962
Breder CD, Dewitt D, Kraig RP (1995) Characterization of inducible cyclooxygenase in rat brain. J Comp Neurol 355:296–315
Tocher DR (2015) Omega-3 long-chain polyunsaturated fatty acids and aquaculture in perspective. Aquaculture 449:94–107
Bell JG, Sargent JR (2003) Arachidonic acid in aquaculture feeds: current status and future opportunities. Aquaculture 218:491–499
Izquierdo MS (1996) Essential fatty acid requirements of cultured marine fish larvae. Aquac Nutr 2:183–191
Ghioni C, Porter AEA, Taylor GW, Tocher DR (2002) Metabolism of 18:4n-3 (stearidonic acid) and 20:4n-3 in salmonid cells in culture and inhibition of the production of prostaglandin F2α (PGF2α) from 20:4n-6 (arachidonic acid). Fish Physiol Biochem 27:81–96
Castell JD, Bell JG, Tocher DR, Sargent JR (1994) Effects of purified diets containing different combinations of arachidonic and docosahexaenoic acid on survival, growth and fatty acid composition of juvenile turbot (Scophthalmus maximus). Aquaculture 128:315–333
Atalah E, Hernández-Cruz CM, Benítez-Santana T, Ganga R, Roo J, Izquierdo M (2011) Importance of the relative levels of dietary arachidonic acid and eicosapentaenoic acid for culture performance of gilthead seabream (Sparus aurata) larvae. Aquac Res 42:1279–1288
Yuan Y, Li S, Mai K, Xu W, Zhang Y, Ai Q (2015) The effect of dietary arachidonic acid (ARA) on growth performance, fatty acid composition and expression of ARA metabolism-related genes in larval half-smooth tongue sole (Cynoglossus semilaevis). Br J Nutr 113:1518–1530
Montero D, Terova G, Rimoldi S, Betancor MB, Atalah E, Torrecillas S, Caballero MJ, Zamorano MJ, Izquierdo M (2015) Modulation of the expression of components of the stress response by dietary arachidonic acid in European sea bass (Dicentrarchus labrax) larvae. Lipids 50:1029–1041
Glencross BD, Rutherford N (2011) A determination of the quantitative requirements for docosahexaenoic acid for juvenile barramundi (Lates calcarifer). Aquac Nutr 17:e536–e548
Nichols PD, Glencross B, Petrie JR, Singh SP (2014) Readily available sources of long-chain omega-3 oils: is farmed Australian seafood a better source of the good oil than wild-caught seafood? Nutrients 6:1063–1079
Brown PB, Hart SD (2011) Soybean oil and other n-6 polyunsaturated fatty acid-rich vegetable oils. In: Turchini GM, Ng WK, Tocher DR (eds) Fish oil replacement and alternative lipid sources in aquaculture feeds. CRC Press, Taylor and Francis group, Boca Raton
Salini MJ, Irvin SJ, Bourne N, Blyth D, Cheers S, Habilay N, Glencross BD (2015) Marginal efficiencies of long chain-polyunsaturated fatty acid use by barramundi (Lates calcarifer) when fed diets with varying blends of fish oil and poultry fat. Aquaculture 449:48–57
Glencross BD, Curnow J, Hawkins W, Kissil GWM, Peterson D (2003) Evaluation of the feed value of a transgenic strain of the narrow-leaf lupin (Lupinus angustifolius) in the diet of the marine fish, Pagrus auratus. Aquac Nutr 9:197–206
Wade N, Skiba-Cassy S, Dias K, Glencross B (2014) Postprandial molecular responses in the liver of the barramundi, Lates calcarifer. Fish Physiol Biochem 40:427–443
Folch J, Lees M, Sloane-Stanley G (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Christie WW (2003) Lipid analysis, isolation, separation, identification and structural analysis of lipids. PJ Barnes and Associates, Bridgewater
Ackman RG (2002) The gas chromatograph in practical analysis of common and uncommon fatty acids for the 21st century. Anal Chim Acta 465:175–192
Salini MJ, Turchini GM, Wade N, Glencross BD (2015) Rapid effects of essential fatty acid deficiency on growth and development parameters and transcription of key fatty acid metabolism genes in juvenile barramundi Lates calcarifer. Br J Nutr 114:1784–1796
Marshall OJ (2004) PerlPrimer: cross-platform, graphical primer design for standard, bisulphite and real-time PCR. Bioinformatics 20:2471–2472
De Santis C, Smith-Keune C, Jerry DR (2011) Normalizing RT-qPCR data: are we getting the right answers? An appraisal of normalization approaches and internal reference genes from a case study in the finfish Lates calcarifer. Mar Biotechnol 13:170–180
Maynard LA, Loosli JK (1979) Animal nutrition. McGraw-Hill Book Co., New York
R Core Team (2012) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Cottin SC, Sanders TA, Hall WL (2011) The differential effects of EPA and DHA on cardiovascular risk factors. Proc Nutr Soc 70:215–231
Aarsland A, Lundquist M, Børretsen B, Berge R (1990) On the effect of peroxisomal β-oxidation and carnitine palmitoyltransferase activity by eicosapentaenoic acid in liver and heart from rats. Lipids 25:546–548
Thomassen MS, Rein D, Berge GM, Østbye TK, Ruyter B (2012) High dietary EPA does not inhibit Δ5 and Δ6 desaturases in Atlantic salmon (Salmo salar L.) fed rapeseed oil diets. Aquaculture 360–361:78–85
Trushenski J, Schwarz M, Bergman A, Rombenso A, Delbos B (2012) DHA is essential, EPA appears largely expendable, in meeting the n-3 long-chain polyunsaturated fatty acid requirements of juvenile cobia Rachycentron canadum. Aquaculture 326–329:81–89
Villalta M, Estevez A, Bransden MP, Bell JG (2008) Effects of dietary eicosapentaenoic acid on growth, survival, pigmentation and fatty acid composition in Senegal sole (Solea senegalensis) larvae during the Artemia feeding period. Aquac Nutr 14:232–241
Watanabe T, Arakawa T, Takeuchi T, Satoh S (1989) Comparison between eicosapentaenoic and docosahexaenoic acids in terms of essential fatty acid efficiency in Juvenile striped jack Pseudocaranx dentex. Nippon Suisan Gakk 55:1989–1995
Bell JG, Tocher D, MacDonald F, Sargent J (1995) Effects of dietary borage oil [enriched in γ-linolenic acid,18:3(n-6)] or marine fish oil [enriched in eicosapentaenoic acid,20:5(n-3)] on growth, mortalities, liver histopathology and lipid composition of juvenile turbot (Scophthalmus maximus). Fish Physiol Biochem 14:373–383
Takeuchi T, Toyota M, Satoh S, Watanabe T (1990) Requirement of juvenile red seabream Pagrus major for eicosapentaenoic and docosahexaenoic acids. Nippon Suisan Gakk 56:1263–1269
Furuita H, Takeuchi T, Toyota M, Watanabe T (1996) EPA and DHA requirements in early juvenile red sea bream using HUFA enriched Artemia nauplii. Fish Sci 62:246–251
Bransden MP, Butterfield GM, Walden J, McEvoy LA, Bell JG (2005) Tank colour and dietary arachidonic acid affects pigmentation, eicosanoid production and tissue fatty acid profile of larval Atlantic cod (Gadus morhua). Aquaculture 250:328–340
Alves Martins D, Rocha F, Martínez-Rodríguez G, Bell G, Morais S, Castanheira F, Bandarra N, Coutinho J, Yúfera M, Conceição LE (2012) Teleost fish larvae adapt to dietary arachidonic acid supply through modulation of the expression of lipid metabolism and stress response genes. Br J Nutr 108:864–874
Fountoulaki E, Alexis MN, Nengas I, Venou B (2003) Effects of dietary arachidonic acid (20:4n-6), on growth, body composition, and tissue fatty acid profile of gilthead bream fingerlings (Sparus aurata L.). Aquaculture 225:309–323
Villalta M, Estévez A, Bransden MP (2005) Arachidonic acid enriched live prey induces albinism in Senegal sole (Solea senegalensis) larvae. Aquaculture 245:193–209
Estévez A, McEvoy LA, Bell JG, Sargent JR (1999) Growth, survival, lipid composition and pigmentation of turbot (Scophthalmus maximus) larvae fed live-prey enriched in arachidonic and eicosapentaenoic acids. Aquaculture 180:321–343
Norambuena F, Morais S, Emery JA, Turchini GM (2015) Arachidonic acid and eicosapentaenoic acid metabolism in juvenile Atlantic salmon as affected by water temperature. PLoS One 10:e0143622
Rosenlund G, Corraze G, Izquierdo M, Torstensen BE (2011) The effects of fish oil replacement on nutritional and organoleptic qualities of farmed fish. In: Turchini GM, Ng WK, Tocher D (eds) Fish oil replacement and alternative lipid sources in aquaculture feeds. CRC Press, Taylor and Francis group, Boca Raton
Norambuena F, Morais S, Estévez A, Bell JG, Tocher DR, Navarro JC, Cerdà J, Duncan N (2013) Dietary modulation of arachidonic acid metabolism in Senegalese sole (Solea Senegalensis) broodstock reared in captivity. Aquaculture 372–375:80–88
Glencross BD, Hawkins WE, Curnow JG (2003) Restoration of the fatty acid composition of red seabream (Pagrus auratus) using a fish oil finishing diet after grow-out on plant oil based diets. Aquac Nutr 9:409–418
Rangel-Huerta OD, Aguilera CM, Mesa MD, Gil A (2012) Omega-3 long-chain polyunsaturated fatty acids supplementation on inflammatory biomakers: a systematic review of randomised clinical trials. Br J Nutr 107:S159–S170
Badawi AF, El-Sohemy A, Stephen LL, Ghoshal AK, Archer MC (1998) The effect of dietary n-3 and n-6 polyunsaturated fatty acids on the expression of cyclooxygenase 1 and 2 and levels of p21ras in rat mammary glands. Carcinogenesis 19:905–910
Sinclair A, O’Dea K, Naughton J (1983) Elevated levels of arachidonic acid in fish from northern Australian coastal waters. Lipids 18:877–881
Sijben JWC, Calder PC (2007) Differential immunomodulation with long-chain n-3 PUFA in health and chronic disease. Proc Nutr Soc 66:237–259
Montero D, Benitez-Dorta V, Caballero MJ, Ponce M, Torrecillas S, Izquierdo M, Zamorano MJ, Manchado M (2015) Dietary vegetable oils: effects on the expression of immune-related genes in Senegalese sole (Solea senegalensis) intestine. Fish Shellfish Immunol 44:100–108
Zuo R, Mai K, Xu W, Turchini G, Ai Q (2015) Dietary ALA, but not LNA, increase growth, reduce inflammatory processes, and increase anti-oxidant capacity in the marine finfish Larimichthys crocea. Lipids 50:149–163
Acknowledgments
The authors would like to thank and acknowledge the technical assistance provided by CSIRO Agriculture staff including David Blyth, Natalie Habilay, Simon Irvin, and David Poppi at the Bribie Island Research Centre (BIRC), Queensland, Australia. This research received no specific grant from any funding agency, commercial or not-for-profit sectors. However, we would like to acknowledge the CSIRO Agriculture for financial assistance. There are no conflicts of interest to report.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Salini, M.J., Wade, N.M., Araújo, B.C. et al. Eicosapentaenoic Acid, Arachidonic Acid and Eicosanoid Metabolism in Juvenile Barramundi Lates calcarifer . Lipids 51, 973–988 (2016). https://doi.org/10.1007/s11745-016-4167-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-016-4167-4