Abstract
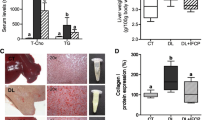
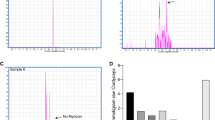
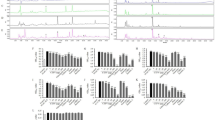
Previously, we found that Celastrus orbiculatus Thunb. (COT) decreases athero-susceptibility in lipoproteins and the aorta of guinea pigs fed a high-fat diet, and increases high-density lipoprotein (HDL). In the present study, we investigated the effect of COT in reducing lipid accumulation and promoting reverse cholesterol transport (RCT) in vivo and vitro. Healthy male mice were treated with high-fat diet alone, high-fat diet with COT (10.0 g/kg/d), or general fodder for 6 weeks. Serum levels of total cholesterol (TC), triglyceride (TG), HDL-C, non-HDL-C, and 3H-cholesterol in plasma, liver, bile, and feces were determined. Pathological changes and the levels of TC and TG in liver were examined. The expression of hepatic genes and protein associated with RCT were analyzed. COT administration reduced lipid accumulation in the liver, ameliorated the pathological changes, and lessened liver injury, the levels of TG, TC, and non-HDL-C in plasma were decreased significantly, and COT led to a significant increase in plasma HDL-C and apolipoprotein A (apoA1). 3H-cholesterol in plasma, liver, bile, and feces was also significantly increased in COT-treated mice compared to controls. Both mRNA and protein expression of SRB1, CYP7A1, LDLR, ATP-binding cassette transporters ABCA1, ABCG5, and LXRα were improved in COT-treated mice. An in vitro isotope tracing experiment showed that COT and its bioactive ingredients, such as celastrol, ursolic acid, oleanolic acid, and quercetin, significantly increased the efflux of 3H-cholesterol. They also increased the expression of SRB1, ABCA1, and ABCG1 significantly in macrophages. Our findings provided a positive role of COT in reducing lipid accumulation by promoting RCT. These effects may be achieved by activating the SRB1 and ABC transporter pathway and promoting cholesterol metabolism via the CYP7A1 pathway in vivo. The effective ingredients in vitro are celastrol, ursolic acid, oleanolic acid, and quercetin.








Similar content being viewed by others
Abbreviations
- COT:
-
Celastrus orbiculatus Thunb
- RCT:
-
Reverse cholesterol transport
- HDL-C:
-
High-density lipoprotein cholesterol
- apoA1:
-
Apolipoprotein A
- TC:
-
Total cholesterol
- TG:
-
Triglyceride
- SR-B1:
-
Scavenger receptor B1
- ABCA1:
-
ATP-binding cassette transporter G1
- ABCG1:
-
ATP-binding cassette transporter A1
- CE:
-
Cholesterol ester
- ABCG5:
-
ATP-binding cassette transporters G5
- ABCG8:
-
ATP-binding cassette transporters G8
- CYP7A1:
-
Cholesterol 7-alpha hydroxylase
- SR-B1:
-
Scavenger receptor class B type 1
- LXR:
-
Liver X receptor
- TCMs:
-
Traditional Chinese medicines
- AS:
-
Atherosclerosis
- SDS-PAGE:
-
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- CL:
-
Celastrol
- RT:
-
Rutin
- QC:
-
Quercetin
- KF:
-
Kaempferol
- OA:
-
Oleanolic acid
- UA:
-
Ursolic acid
- RT-PCR:
-
Real time PCR
- LDL-R:
-
Low-density lipoprotein receptor
- LSC:
-
Liquid scintillation counter
References
Assmann G, Cullen P, Jossa F, Lewis B, Mancini M (1999) Coronary heart disease: reducing the risk: the scientific background to primary and secondary prevention of coronary heart disease. A worldwide view. International task force for the prevention of coronary heart disease. Arterioscler Thromb Vasc Biol 19:1819–1824
Lewis GF, Rader DJ (2005) New insights into the regulation of HDL metabolism and reverse cholesterol transport. Circ Res 96:1221–1232
Wang X, Collins HL, Ranalletta M, Fuki IV, Billheimer JT, Rothblat GH, Tall AR, Rader DJ (2007) Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo. J Clin Invest 117:2216–2244
Rader DJ, Alexander ET, Weibel GL, Billheimer J, Rothblat GH (2009) The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J Lipid Res 50(Suppl):S189–S194
Naik SU, Wang X, DaSilva JS, Jaye M, Macphee CH, Reilly MP, Billheimer JT, Rothblat GH, Rader DJ (2006) Pharmacological activation of liver X receptors promotes reverse cholesterol transport in vivo. Circulation 113:90–97
Moon M, Lee MS, Kim CT, Kim Y (2007) Dietary chitosan enhances hepatic CYP7A1 activity and reduces plasma and liver cholesterol concentrations in diet-induced hypercholesterolemia in rats. Nutr Res Pract 1:175–179
Cavelier C, Lorenzi I, Rohrer L, von Eckardstein A (2006) Lipid efflux by the ATP-binding cassette transporters ABCA1 and ABCG1. Biochim Biophys Acta 1761:655–666
Beltowski J, Wojcicka G, Jamroz-wisniewska A (2009) Adverse effects of statins-mechanisma and consequences. Curr Drug Saf 4:209–228
Qian YY, Zhang H, Hou Y, Yuan L, Li GQ, Guo SY, Hisamits T, Liu YQ (2012) Celastrus orbiculatus extract inhibits tumor angiogenesis by targeting vascular endothelial growth factor signaling pathway and shows potent antitumor activity in hepatocarcinomas in vitro and in vivo. Chin J Integr Med 18:752–760
Jin HZ, Hwang BY, Kim HS, Lee JH, Kim YH, Lee JJ (2002) Antiinflammatory constituents of Celastrus orbiculatus inhibit the NF-kappaB activation and NO production. J Nat Prod 65:89–91
Zhang Y, Si YH, Yao ST, Yang NN, Song GH, Sang H, Zu DD, Xu X, Wang J, Qin SC (2013) Celastrus orbiculatus Thunb. Decreases athero-susceptibility in lipoproteins and the aorta of guinea pigs fed high fat diet. Lipids 48:619–631
Qin L, Qin XP, Wang Z, Zhu BY, Liao DF (2006) Effect of pravastatin on cholesteryl esters in foam cells and the relation with caveolin-1. Acta Physiol Sin 58:47–52 (Chinese, English abstract)
Sherman MP, Aeberhard EE, Wong VZ, Griscavage JM, Ignarro LJ (1993) Pyrrolidine dithiocar -bamate inhibits induction of nitric oxide synthase activity in rat alveolar macrophages. Biochem Biophys Res Commun 191:1301–1308
Chapman MJ, Mills GL, Ledford JH (1975) The distribution and partial characterization of the serum apolipoproteins in the guinea pig. Biochem J 149:423–436
Yu Y, Si YH, Song GH, Luo T, Wang JF, Qin SC (2011) Ethanolic extract of propolis promotes reverse cholesterol transport and the expression of ATP-binding cassette transporter A1 and G1 in mice. Lipids 46:805–811
Zhang Y, Da Silva JR, Reilly M, Billheimer JT, Rothblat GH, Rader DJ (2005) Hepatic expression of scavenger receptor class B type I (SR-BI) is a positive regulator of macrophage reverse cholesterol transport in vivo. J Clin Invest 115:2870–2874
Van Eck M, Pennings M, Hoekstra M, Out R, Van Berkel TJ (2005) Scavenger receptor BI and ATP-binding cassette transporter A1 in reverse cholesterol transport and atherosclerosis. Curr Opin Lipidol 16:307–315
Yvan-Charvet L, Wang N, Tall AR (2010) Role of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and immune responses. Arterioscler Thromb Vasc Biol 30:139–143
Trigatti BL, Krieger M, Rigotti A (2003) Influence of the HDL receptor SR-BI on lipoprotein metabolism and atherosclerosis. Arterioscler Thromb Vasc Biol 23:1732–1738
Chiang JY (2009) Bile acids: regulation of synthesis. J Lipid Res 50:1955–1966
Brown MS, Goldstein JL (1986) A receptor-mediated pathway for cholesterol homeostasis. Science 232:34–47
Schmitz G, Langmann T, Heimerl S (2001) Role of ABCG1 and other ABCG family members in lipid metabolism. J Lipid Res 42:1513–2018
Malerod L, Juvet L, Hanssen-Bauer A, Eskild W, Berg T (2002) Oxysterol-activated LXRalpha/RXR induces SR-BI-promoter activity in hepatoma cells and preadipocytes. Biochem Biophys Res Commun 299:916–923
Bhaskar S, Kumar KS, Krishnan K, Antony H (2013) Quercetin alleviates hypercholes-terolemic diet induced inflammation during progression and regression of atherosclerosis in rabbits. Nutrition 29:219–229
Xiao HB, Lu XY, Sun ZL, Zhang HB (2011) Kaempferol regulates OPN-CD44 pathway to inhibit the athero-genesis of apolipoprotein E deficient mice. Toxicol Appl Pharmacol 257:405–411
Zhang J, Liang R, Wang L, Yan R, Hou R, Gao S, Yang B (2013) Effects of an aqueous extract of Crataegus pinnatifida Bge. var. major N.E.Br. fruit on experimental atherosclerosis in rats. J Ethnopharmacol 48:63–69
Boots AW, Haenen GR, Bast A (2008) Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol 585:325–337
Sang WK, Min SK, Hyun SK, Yunho K, Daekeun S, Jung Han YP, Young HK (2013) Celastrol attenuates adipokine resistin-associated matrix interaction and migration of vascular smooth muscle cells. J Cell Biochem 114:398–408
Buus NH, Hansson NC, Rodriguez-Rodriguez R, Stankevicius E, Andersen MR, Simonsen U (2011) Antiatherogenic effects of oleanolic acid in apolipoprotein E knockout mice. Eur J Pharmacol 670:519–526
Chang YC, Lee TS, Chiang AN (2012) Quercetin enhances ABCA1 expression and cholesterol efflux through a p38-dependent pathway in macrophages. J Lipid Res 53:1840–1850
Park MY, Ji GE, Sung MK (2012) Dietary kaempferol suppresses inflammation of dextran sulfate sodium-induced colitis in mice. Dig Dis Sci 57:355–363
Calderón-Montaño JM, Burgos-Morón E, Pérez-Guerrero C, López-Lázaro M (2011) A review on the dietary flavonoid kaempferol. Mini Rev Med Chem 11:298–344
Bischoff SC (2008) Quercetin: potentials in the prevention and therapy of disease. Curr Opin Clin Nutr Metab Care 11:733–740
Rivera L, Morón R, Sánchez M, Zarzuelo A, Galisteo M (2008) Quercetin ameliorates metabolic syndrome and improves the inflammatory status in obese Zucker rats. Obesity (Silver Spring) 16:2081–2087
Kleemann R, Verschuren L, Morrison M, Zadelaar S, van Erk MJ, Wielinga PY, Kooistra T (2011) Anti-inflammatory, antiproliferative and anti-atherosclerotic effects of quercetin in human in vitro and in vivo models. Atherosclerosis 218:44–52
Juźwiak S, Wójcicki J, Mokrzycki K, Marchlewicz M, Białecka M, Wenda-Rózewicka L, Gawrońska–Szlarz B, Droździk M (2005) Effect of quercetin on experimental hyperlipidemia and atherosclerosis in rabbits. Pharmacol Rep 57:604–609
Auger C, Teissedre PL, Gérain P, Lequeux N, Bornet A, Serisier S, Besançon P, Caporiccio B, Cristol JP, Rouanet JM (2005) Dietary wine phenolics catechin, quercetin, and resveratrol efficiently protect hypercholesterolemic hamsters against aortic fatty streak accumulation. J Agric Food Chem 53:2015–2021
Chirumbolo S (2010) The role of quercetin, flavonols and flavones in modulating inflammatory cell function. Inflamm Allergy Drug Targets 9:263–285
Li H, Wang QJ, Zhu DN, Yang Y (2008) Reinioside C, a triterpene saponin of Polygala aureocauda Dunn, exerts hypolipidemic effect on hyperlipidemic mice. Phytother Res 22:159–164
Kannaiyan R, Shanmugam MK, Sethi G (2011) Molecular targets of celastrol derived from Thunder of God Vine: potential role in the treatment of inflammatory disorders and cancer. Cancer Lett 303:9–20
Somova LO, Nadar A, Rammanan P, Shode FO (2003) Cardiovascular, antihyperlipidemic and antioxidant effects of oleanolic and ursolic acids in experimental hypertension. Phytomedicine 10:115–121
Liu J (1995) Pharmacology of oleanolic acid and ursolic acid. J Ethnopharmacol 49:57–68
Acknowledgments
This research was supported by financial donations from the Taishan Scholars Foundation of Shandong Province (200867), University of Science and Technology Plan Projects Fund of Shandong Province (J121M01), Taian City Technology Development Plan (201440774-03), Key development projects of Shandong province (2015GSF119008), and the Shandong Provincial Natural Science Fund (ZR2013HL063, ZR2013HQ014).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interest exist.
Additional information
Y. Zhang, Y. Si contributed equally to this study.
About this article
Cite this article
Zhang, Y., Si, Y., Zhai, L. et al. Celastrus Orbiculatus Thunb. Reduces Lipid Accumulation by Promoting Reverse Cholesterol Transport in Hyperlipidemic Mice. Lipids 51, 677–692 (2016). https://doi.org/10.1007/s11745-016-4145-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-016-4145-x