Abstract
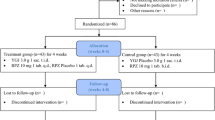
Nonerosive reflux disease (NERD) is a gastrointestinal disorder that leads to symptoms such as heartburn and regurgitation without visible esophageal mucosal injury, and it is treated with proton-pump inhibitors (PPIs). CHETOGERD® gel or orosoluble (oro) formulations—an association of natural active ingredient: hyaluronic acid, altea, malva, apple active TM, Aloe vera, l-triptophan, calcium gluconate, sodium bicarbonate, Musa paradisiaca)—may be an alternative or a coadjutant treatment in patients with NERD. The aim of the study was to evaluate, prospectively, the efficacy of CHETOGERD® gel and oro in inducing symptom’s reduction or remission, in consecutive patients with NERD. Patients were divided in two groups and treated with CHETOGERD® gel or CHETOGERD® oro, 3 sachets/day for 3 months, decreased to 1 sachet/day for other 3 months. Symptoms were evaluated at baseline, 3 and 6 months using the reflux disease questionnaire (RDQ). Symptoms’ remission was defined as reduction of retrosternal pain or burning, epigastric pain or burning, regurgitation and acid sensation in mouth. Frequency, distribution analyses and non-parametric tests were used for the statistical analysis. Results were considered statistically significant for p values < 0.05. Four hundred and twenty-three patients (M/F 240/183; mean age 50 years) were diagnosed with NERD and were consecutively enrolled. 146 patients underwent therapy with CHETOGERD® gel, while 277 were treated with CHETOGERD® oro. 108 patients from the first group and 172 patients from the second group completed follow up at 3 months, while 100 patients from each group completed follow-up at 6 months. Both formulations were able to significantly reduce the frequency and intensity of symptoms analysed with RDQ. No adverse events were reported. CHETOGERD® gel and oro are two valid alternatives to control symptoms in patients with nonerosive reflux disease.


Similar content being viewed by others
References
Vakil N, van Zanten SV, Kahrilas P et al (2006) The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 101(8):1900–1920
Savarino E, Zentilin P, Savarino V (2013) NERD: an umbrella term including heterogeneous subpopulations. Nat Rev Gastroenterol Hepatol 10(6):371–380. https://doi.org/10.1038/nrgastro.2013.50
El-Serag HB (2008) Epidemiology of non-erosive reflux disease. Digestion 78(Suppl 1):6–10. https://doi.org/10.1159/000151249
Dutta U, Moayyedi P (2013) Management of reflux-related symptoms. Best Pract Res Clin Gastroenterol 27(3):387–400. https://doi.org/10.1016/j.bpg.2013.06.004
Gyawali CP, Kahrilas PJ, Savarino E et al (2018) Modern diagnosis of GERD: the Lyon Consensus. Gut 67(7):1351–1362. https://doi.org/10.1136/gutjnl-2017-314722
Katz PO, Gerson LB, Vela MF (2013) Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol 108(3):308–328. https://doi.org/10.1038/ajg.2012.444
Trifan A, Stanciu C, Girleanu I et al (2017) Proton pump inhibitors therapy and risk of Clostridium difficile infection: systematic review and meta-analysis. World J Gastroenterol 23(35):6500–6515. https://doi.org/10.3748/wjg.v23.i35.6500
Lambert AA, Lam JO, Paik JJ et al (2015) Risk of community-acquired pneumonia with outpatient proton-pump inhibitor therapy: a systematic review and meta-analysis. PLoS ONE 10(6):e0128004. https://doi.org/10.1371/journal.pone.0128004
Thong BKS, Ima-Nirwana S, Chin KY (2019) Proton pump inhibitors and fracture risk: a review of current evidence and mechanisms involved. Int J Environ Res Public Health 16(9):34–37. https://doi.org/10.3390/ijerph16091571
Armstrong D, Bennett JR, Blum AL et al (1996) The endoscopic assessment of esophagitis: a progress report on observer agreement. Gastroenterology 111(1):85–92. https://doi.org/10.1053/gast.1996.v111.pm8698230
Pace F, Scarlata P, Casini V et al (2008) Validation of the reflux disease questionnaire for an Italian population of patients with gastroesophageal reflux disease. Eur J Gastroenterol Hepatol 20(3):187–190. https://doi.org/10.1097/MEG.0b013e3282f246b2
Tack J, Becher A, Mulligan C et al (2012) Systematic review: the burden of disruptive gastro-oesophageal reflux disease on health-related quality of life. Aliment Pharmacol Ther 35(11):1257–1266. https://doi.org/10.1111/j.1365-2036.2012.05086.x
Dossett ML, Cohen EM, Cohen J (2017) Integrative medicine for gastrointestinal disease. Prim Care 44(2):265–280. https://doi.org/10.1016/j.pop.2017.02.002
Savarino V, Pace F, Scarpignato C et al (2017) Randomised clinical trial: mucosal protection combined with acid suppression in the treatment of non-erosive reflux disease—efficacy of Esoxx, a hyaluronic acid-chondroitin sulphate based bioadhesive formulation. Aliment Pharmacol Ther 45(5):631–642. https://doi.org/10.1111/apt.13914
Salehi M, Karegar-Borzi H, Karimi M et al (2017) Medicinal Plants for management of gastroesophageal reflux disease: a review of animal and human studies. J Altern Complement Med 23(2):82–95. https://doi.org/10.1089/acm.2016.0233
Panahi Y, Khedmat H, Valizadegan G et al (2015) Efficacy and safety of Aloe vera syrup for the treatment gastroesophageal reflux disease: a pilot randomized positive-controlled trial. J Tradit Chin Med 35(6):632–636. https://doi.org/10.1016/s0254-6272(15)30151-5
Pereira Rde S (2006) Regression of gastroesophageal reflux disease symptoms using dietary supplementation with melatonin, vitamins and aminoacids: comparison with omeprazole. J Pineal Res 41(3):195–200. https://doi.org/10.1111/j.1600-079X.2006.00359.x
Alese MO, Adewole SO, Akinwunmi KF et al (2017) Aspirin-induced gastric lesions alters EGFR and PECAM-1 immunoreactivity in Wistar rats: modulatory action of flavonoid fraction of Musa paradisiaca. Open Access Maced J Med Sci 5(5):569–577. https://doi.org/10.3889/oamjms.2017.058
Acknowledgements
The authors received no financial support for the research or publication of this article.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
Guarantor of the article: Professor Dino Vaira. Dino Vaira conceived the study. Dino Vaira and Giulia Fiorini included patients in the study. Matteo Pavoni Laura Saccomanno and Ilaria M. Saracino performed data management and statitstical analysis. Ilaria M. Saracino, Giulia Fiorini, Matteo Pavoni and Laura Saccomanno wrote the manuscript. All the authors critically reviewed the manuscript and approved the final version of this manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflict of interest to disclose.
Statement of human and animal rights
The study was carried out according to Good Clinical practice guidelines.
Informed consent
All patients signed an informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fiorini, G., Saracino, I.M., Pavoni, M. et al. Efficacy of a new nutraceutical formulation (CHETOGERD®) in patients with nonerosive reflux disease (NERD): a prospective observational study. Intern Emerg Med 15, 1265–1269 (2020). https://doi.org/10.1007/s11739-020-02309-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-020-02309-z