Abstract
The boundary between beneficial and phytotoxic levels of selenium (Se) is narrow, and both induce alteration in plant growth and their physiology. In this study, the influence of two Se forms (selenite or selenate) with different concentrations (2–80 µM) on cucumber plants was investigated. The toxicity threshold for selenate and selenite was determined at the concentrations of 80 and 20 µM, respectively. In the Se-exposed plants, the growth-promoting effect was found at 6 µM of selenite and at 6–20 µM of selenate. The root activity considerably increased with increasing selenite concentrations suggesting the upregulation of mitochondrial dehydrogenases activity. Selenite treatment also impaired photosynthetic pigments accumulation and chlorophyll fluorescence parameters. Moreover, Se exerted a dual effect on lipid peroxidation in roots: at low concentrations it inhibited this process, whereas at high concentrations it enhanced the accumulation of harmful lipid peroxides. Under low Se concentrations (<10 µM), the accumulation of Se in shoots was similar in the presence of selenate and selenite. When Se concentration was >10 µM, the accumulation of Se in shoots was greater in selenate-exposed than selenite-exposed plants. However, in the roots the Se concentrations were always higher after selenite exposure comparing to selenate. The N level in plants was generally maintained constant, while the remaining macronutrients (especially K, P, and S) concentrations were significantly changed depending on the form and concentrations of Se. These results imply that an application of either selenate or selenite at concentrations <10 µM may be potentially used for biofortification of cucumber with Se and changes in plant macronutrient contents are not expected under these conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Selenium (Se) is an essential micronutrient for animals, humans, and some microorganisms (Germ et al. 2007). According to Hamilton (2004), it has three levels of biological activity: (1) trace concentrations are necessary for normal growth and development; (2) moderate concentrations can be stored to maintain homeostatic functions; and (3) high concentrations can cause toxic effects. Since either Se deficiency or excess in the human diet can have serious implications for health, this element is often labelled as a ‘double-edged sword’. Selenium is incorporated into the food chain mainly through crop plants and for that reason the Se status of the food chain is strictly dependent on the Se level in the soil, as well as in the edible parts of plants (Hartikainen 2005).
The deficiency and toxicity problems associated with Se may be alleviated through the use of plants, because all plant species are able to take up, accumulate and volatilise Se. Although during the last two decades, the physiological role of Se in plants has been studied by many researches, Se has not been confirmed as an essential nutrient in higher plants, and it is thought that the essential Se metabolism may have gotten lost in this taxonomic group (Valdez Barillas et al. 2011). However, there is increasing evidence that Se at relatively low concentrations is a beneficial element for plants, as an antioxidant and a growth-promoting agent (Garcia-Bañuelos et al. 2011). Therefore, this element, together with aluminium (Al), cobalt (Co), silicon (Si), sodium (Na) and vanadium (V), is included to the group of “beneficial” elements (Kopsell and Kopsell 2007). Currently, the investigation is directed to elucidate the specific physiological and biochemical mechanisms that underlie the positive or toxic effects of Se in plant organisms.
Plants take up Se from the soil solution primarily as the two main oxidised, inorganic forms: selenate (Se VI) and selenite (Se IV). Selenate directly competes with sulphate for uptake by plants since it is transported across the plasmalemma by high-affinity sulphate transporters, whereas selenite is probably transported by phosphate transporters (White and Broadley 2009). Selenate as well as selenite, are metabolised by the same pathway as their sulphur (S) analogues, leading to the incorporation of Se in all S metabolites, including proteins and other S compounds. This non-specific Se substitution instead of S in the S-containing compounds is the main cause of Se phytotoxicity. However, there are differences between uptake, transport, distribution and biological activity of individual Se forms (Terry et al. 2000).
The regulation of the uptake and translocation of some nutrients by Se is thought to be an important mechanism to reactivate antioxidants, reduce the reactive oxygen species (ROS) overproduction and increase plant tolerance to environmental stresses (Feng et al. 2013). The impact of Se on the uptake and assimilation of S in plants has been intensively studied due to chemical similarity of both elements (White et al. 2004). Nevertheless, the information about the influence of Se on the uptake and accumulation of other essential elements by plants is still insufficient, especially that Se biofortification may influence overall nutrient balance of a plant. Therefore, the main objective of this study was to compare the effect of increasing selenite or selenate concentrations on the growth, some physiological parameters, as well as macronutrients and Se accumulation in cucumber plants in terms of the potential use of this species for biofortification.
Materials and methods
Plant material, growth conditions and experimental design
The seeds of cucumber (Cucumis sativus L.) cv. Polan F1 were sown onto wet quartz sand and germinated at 25 °C for 7–8 days. After germination, the best-developed seedlings of uniform size were transferred to 1 L glass jars (two plants each) containing 1.5-fold concentrated Hoagland’s II nutrient solution (Hoagland and Arnon 1950). The pH of the nutrient solution was adjusted to 5.5. Then, the growth medium was differentiated in regard to the form and concentration of Se: 2, 4, 6, 10, 20, 30, 40, 60 or 80 µM Se applied as selenate (Na2SeO4) or 2, 4, 6, 10, 20, 30, 40 or 60 µM applied as selenite (Na2SeO3). On the basis of the results from preliminary experiments, we excluded from the study the 80 µM selenite concentration due to plants dying under these conditions. The control plants were grown without the addition of Se. The cucumbers were cultivated in a controlled-climate chamber (Sanyo, model MRL 350HT) for 14 days under the following conditions: photosynthetic photon flux density of 270 µmol m−2 s−1, 14-h day length, temperature of 25/20 °C (day/night) and relative humidity of 60–65 %. The nutrient solution was aerated for 15 min every 2 days using an aquarium air pump and replenished when required. The pH of the medium was measured every 2 days during plant cultivation and was adjusted to pH 5.5, if necessary.
Determination of growth parameters
After 14 days from Se addition, the control and Se-treated plants from each jar were harvested, separated into roots and shoots, and the fresh weights (FW) were determined immediately after harvest. The fresh second true leaves were scanned using CI-202 laser areametre (CID Bio-Science, USA) and the leaf area (LA) was expressed in square centimetres (cm2).
Determination of photosynthetic pigments concentration and chlorophyll fluorescence parameters
Chlorophylls (a + b) together with all carotenoids (xanthophyll + carotene) were estimated and calculated by the method given by Lichtenthaler and Wellburn (1983). The samples were collected from the second true leaves and the photosynthetic pigments were extracted from samples by homogenisation with 80 % (v/v) acetone. The absorbance of the resulting solutions was recorded at 646, 663, and 470 nm.
Chlorophyll fluorescence parameters included the minimal (F o) and maximal (F m) level of fluorescence and the maximum quantum yield of photosystem II (PS II; F v/F m, where F v = F m − F o) (Schreiber et al. 1994) and were measured using a Handy PEA fluorimeter (Hansated Instruments, Japan) on the same leaves that were used for extraction of photosynthetic pigments. Cucumber leaves were adapted to darkness for 15 min before the measurements by attaching light-exclusion clips.
Measurement of the root activity by TTC method
TTC (2,3,5-triphenyltetrazolium chloride) has been used in several works to study the vitality of different plant tissues and in this experiment the root activity was measured by the method described by Clemensson-Lindell (1994) with a slight modification. TTC in metabolically active cells is reduced to bright red, water-insoluble formazan. This reduction highly depends on a well-functioning electron transport chain in mitochondrial membranes and a coupling of TTC to cytochrome oxidase (Stūrīte et al. 2005). In brief, the root tips samples about 0.5–1.0 cm length (100 mg) were placed into the test tubes. To each test tube was added 3 mL of 0.6 % (w/v) TTC in 0.05 M phosphate buffer (pH 7.4) containing 0.05 % (v/v) wetting agent (Tween 20). The samples were shaken for 3 h at 30 °C before incubation at 30 °C for 20 h. Then, the samples were washed twice in distilled water (10 mL) and extracted in 7 mL of 95 % (v/v) ethanol in a water bath at 85 °C for 5 min. The absorbance of the extracts was recorded at 490 nm and the root activity was expressed as E490 g−1 FW.
Lipid peroxidation assay
The membrane lipid peroxidation level in root tissues was quantified by measuring thiobarbituric acid reactive substances (TBARS) concentration (Heath and Packer 1968). In brief, 500 mg of fresh tissues were ground in 4.5 mL of 0.1 % (w/v) trichloroacetic acid (TCA), and centrifuged at 10,000 rpm for 10 min. Then, 4 mL of 20 % TCA containing 0.5 % of thiobarbituric acid (TBA) (w/v) was added into 1 mL of the obtained supernatant. The solution of TCA + TBA was enriched with butylated hydroxytoluene (BHT) to avoid non-specific TBARS production. The reaction mixture was heated at 95 °C for 30 min, cooled, and re-centrifuged. The absorbance was measured at 532 and 600 nm. The concentration of TBARS red complexes was calculated from the extinction coefficient of 155 mM−1 cm−1.
Analysis of macronutrients and total Se concentration
The dry plant material was subjected to chemical analyses to determine the concentrations of the following macronutrients in the shoots: total nitrogen (N) by the classic Kjeldahl method; phosphorus (P) by vanadium-molybdate colorimetry; magnesium (Mg) by colorimetry using titanium yellow; potassium (K) and calcium (Ca) by AAS technique (Nowosielski 1974).
For the determination of the Se concentrations, the dry plant material was subjected to the nitric–perchloric acids mineralisation (HNO3–HClO4; 4:1; v/v), after which hydride generation atomic absorption spectroscopy (HG-AAS) was used to determine the total Se concentrations as described previously (Hawrylak-Nowak 2013).
Statistical analyses
The experimental unit consisted of six plants per treatment, and the experiment was repeated three times under the same conditions. The data on FW, LA, photosynthetic pigments concentration and chlorophyll fluorescence parameters were statistically analysed by applying one-way ANOVA to assess merely the responses of plants to Se concentration at its two chemical forms. However, the root activity, MDA concentrations as well as macronutrients and Se concentrations were subjected to two-way ANOVA with chemical form and Se concentration as experimental factors and the results of statistical analysis of these data represent the effect of interaction between these two factors. Significance of differences was assessed using the Tukey’s multiple range test at the confidence level of p < 0.05.
Results
Growth parameters and threshold of Se toxicity
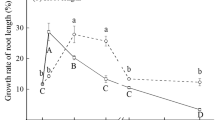
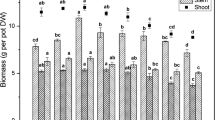
The threshold of Se toxicity, depending on its chemical form, has been designated on the basis of fresh weight (FW) of the plant’s organs and defined as the lowest concentration of Se causing a significant decrease in the shoot or root FW, compared to the control plants. The FW of plant organs decreased significantly if the selenate or selenite concentrations in the nutrient solution reached 80 and 20 µM, respectively (Fig. 1a, b).
If the Se concentration increased, a further decrease in the biomass of roots and shoots occurred. Under the highest concentration of selenate (80 µM), the shoot and root FW decreased by 15 and 21 %, respectively (Fig. 1a), whereas under the highest concentration of selenite (60 µM), the shoot and root FW decreased by 77 and 89 %, respectively (Fig. 1b). Furthermore, under phytotoxic concentrations of Se, along with the reduction in biomass a corresponding reduction of the LA was found (Fig. 1a, b). Compared to the control, the highest Se concentrations used decreased the LA by 21 and 84 % in the presence of selenate and selenite, respectively. Moreover, Se phytotoxicity symptoms included foliar chlorosis (Fig. 2), stunting of shoots, reduced root growth, and were observed mainly in plants grown under selenite exposure. These results demonstrated that selenite is much more toxic for cucumber plants than selenate.
On the other hand, low Se concentrations promoted the growth of cucumber. The growth-promoting effect of Se was the highest at 6–20 µM of selenate (Fig. 1a) and at 6 µM of selenite (Fig. 1b). Under these conditions, a significant increase in the FW of root, shoot and/or LA was found, compared to the control plants.
Concentration of photosynthetic pigments and chlorophyll fluorescence parameters
Selenate treatments ≤40 µM had no significant effects on the concentration of photosynthetic pigments. The higher selenate concentrations disrupted the accumulation of chlorophylls but not carotenoids, where its contents remained at the control level under all selenate concentrations tested (Fig. 3a). Meanwhile, selenite at the concentration of 10 µM already significantly reduced the chlorophyll concentrations, and in the presence of 30 µM of selenite the reduced carotenoids content was found (Fig. 3b). A further increase in the concentration of selenite caused a progressive reduction in the level of photosynthetic pigments, especially chlorophylls. Moreover, it was noticed that chlorophyll b was more affected by toxic Se concentrations than chlorophyll a.
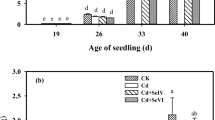
The analysis of chlorophyll fluorescence parameters show that selenate treatments generally did not affect the F o, F m, and F v/F m values (Fig. 4a). However, as shown in Fig. 4b, if selenite concentration was >20 µM, both F m and F v/F m values tended to decrease, whereas F o value increased.
Effects of increased selenate (a) or selenite (b) concentrations in the nutrient solution on the chlorophyll fluorescence parameters in cucumber plants. The values are mean ± SD (n = 12). Different letters for each parameter indicate a significant difference at p < 0.05. No letters mean that the results were not significantly different
The activity of roots and the level of lipid peroxidation of root cell membranes
The root activity, measured by a triphenyl tetrazolium chloride (TTC) test, increased under increasing concentrations of selenite (Fig. 5a). Under the highest selenite dose, the root activities were about tenfold higher than those noted in the control plants. Meanwhile, selenate application caused slighter than selenite variations in the root activity. However, an increase in the activity of roots (by 50 and 63 % in comparison to control) in the presence of 6 and 10 µM of selenate, respectively, was found (Fig. 5a).
Effects of increased selenate or selenite concentrations in the nutrient solution on the activity of roots measured by TTC method (a) and the TBARS concentrations in root tissues (b) of cucumber plants. The values are mean ± SD (n = 6). Different letters for each Se form indicate a significant difference at p < 0.05
Application of Se to the growth media also affected the lipid peroxidation rate in the root cell membranes (Fig. 5b). Selenate treatments at concentrations of 2–60 µM and selenite treatments at concentrations of 2–6 µM significantly lowered lipid peroxidation level in terms of TBARS concentrations as compared to control. As the concentrations of selenite applied increased >20 µM, the TBARS level tended to increase, reaching the highest value at 60 µM of selenite. Although under highest selenate dose (80 µM) the lipid peroxidation increased, but only to the level noted in the control plants.
Macronutrients concentration
Because Se accumulation may influence the nutrient balance of plants, the concentrations of macronutrients were determined in the aboveground cucumber organs. When the growth medium was supplemented with Se, the total N concentrations generally remained at the control level with the exception of significant decrease in N amounts in plants supplemented with 60 µM of selenite (Table 1). Also the Mg concentrations decreased only under a highly phytotoxic selenite concentrations (40 and 60 µM).
The amounts of P were maintained generally at the control level when selenite was applied at low concentrations (2–10 µM) and decreased if selenite concentrations rise, reaching only 30 % of the control value at its highest concentration. Interestingly, a different pattern was observed in the presence of selenate, when its application at concentrations of 2, 6, 40 and 60 µM provoked an increase in P content (by 22–38 %) in a dose independent manner.
The significant decrease in the K levels was noted if selanate or selenite concentration in the growth media passed 6 µM. However, under selenite treatments the decline in K content was higher compared to selenate.
The effects of Se on Ca concentration depended on the chemical form of Se added to the growth media. Whereas selenite treatments between 30 and 60 µM significantly decreased the Ca concentration in the dose-dependent manner, the exposition of plants to selenate resulted in a slightly higher concentration of Ca, but a significant increase was noted only in the presence of 40 and 80 µM Se applied as selenate.
Under low Se treatments (2–6 µM), the S–SO4 concentration remained at the control level. A further increase in the concentration of Se in the selenate form caused a dose-dependent increase in the S–SO4 accumulation, by 30–89 % in comparison to the control. However, in the presence of 20–30 µM Se in the selenite form the S–SO4 concentration declined by 22–40 %, but has risen by 25–64 % after exposure on 40–60 µM of selenite.
Total Se concentrations and Se translocation form roots to shoots
Under low Se concentrations (2–10 µM), the accumulation of Se in shoots of cucumber was similar in the presence of selenate and selenite (Table 2). However, when Se concentration in the growth media was higher than 10 µM, Se accumulation was greater when selenate rather than selenite was applied. For example, when 30 µM Se was added, total Se concentration in shoots was about twofold higher in plants treated selenate in comparison to selenite, and when 60 µM Se was added, total Se concentration in shoots was over threefold higher in plants treated with selenate in comparison to selenite. However, in the root tissues the concentrations of Se were much higher after the application of selenite than selenate, regardless of Se concentration in the growth media. The translocation of Se from roots to shoots was highly dependent on the chemical form of exogenous Se. The TF value (ratio of Se concentration in shoot to root) ranged from 0.93 to 1.38 in selenate-treated plants and was ≤0.2 for those supplied with selenite (Table 2).
Discussion
In this experiment, it was found that Se stimulated the plant growth at low concentrations, but was inhibited at high concentrations, depending on the chemical form of Se. The biomass of cucumber plants decreased if selenate or selenite concentrations in the growth medium reached 80 and 20 µM, respectively. Comparing the values of the toxicity threshold, it is obvious that cucumber plants were not as sensitive to Se, especially in the selenate form, as lettuce (Hawrylak-Nowak 2013) where 20 µM of selenate and 15 µM of selenite significantly reduced the plant’s growth. The toxicity mechanisms of Se excess have been discussed extensively in the literature (Terry et al. 2000 and references therein). In studies performed by Funes-Collado et al. (2013), among edible plants fortified with Se, cabbage showed the greatest tolerance to Se and lettuce and parsley were the most sensitive. Despite the growth reduction, the decrease in the photosynthetic pigments concentration is the primary bioindicator of trace elements phytotoxicity. In our study, a significant decrease in chlorophyll levels appeared at the lower Se concentrations than the reduction of plant biomass or leaf area. This shows that the decrease in the chlorophyll concentrations is a more sensitive indicator of Se phytotoxicity in cucumber than a reduction in plant growth. Moreover, chlorophyll b was more sensitive to the Se stress than chlorophyll a which was found also in spinach plants (Saffaryazdi et al. 2012). On the other hand, in the Se-treated cucumbers, the growth-promoting activity of Se was found at 6 µM of selenite and at 6–20 µM of selenate. The dual effect of Se on plant growth (positive or toxic) dependent on concentrations of Se was also found in other plant species (White et al. 2004; Hajiboland and Amjad 2007; Ríos et al. 2008; Ramos et al. 2011; Saffaryazdi et al. 2012; Hawrylak-Nowak 2013).
Chlorophyll fluorescence parameters (e.g. F o, F m, F v/F m) are commonly used to characterise the primary PSII photochemistry, which is interrelated with the photosynthetic capacity. An increase in F o or a decrease in F m and F v/F m reflects the damage caused by environmental stresses (Zhang et al. 2014). In our experiment, the application of selenate did not influence the chlorophyll fluorescence parameters. Nevertheless, selenite at concentrations >20 µM impaired the values of F o, F m, and F v/F m. Similarly, Valkama et al. (2003) did not find any influence of selenate on barley chlorophyll fluorescence. They suggest that the high selenate dosage had a harmful effect on photosynthesis via changes in activity and/or biosynthesis of enzymes, rather than via alteration of PSII. In the field experiment of Zhang et al. (2014), Se applied as selenite at concentrations of 20–50 g Se ha−1 enhanced photosynthesis rate and the activity of the photosynthetic system in rice plants. Nevertheless, as the concentration of selenite increased >50 g Se ha−1, both the F v/F m and F v/F o ratios tended to decrease.
Our results agree with those obtained in previous works, which reported that increasing concentrations of Se in a growth media can evoke an increase of Se content in crop plants (Broadley et al. 2006). In our experiments, the total Se concentrations in cucumber increased in a dose-dependent manner after Se addition. It is well-known that selenate is more easily transferred from the root to aboveground organs than selenite or organic Se, since much of selenite is retained in the root tissues where it is rapidly transformed into organic Se compounds (Zayed et al. 1998). In our study, the poor translocation of Se applied as selenite also was found, since under selenite exposure plants accumulated great amounts of Se in their roots. Interestingly, the differences in shoot Se accumulation between selenate and selenite-exposed cucumbers appeared if the Se concentration in the nutrient solution exceeded 10 µM and beyond this concentration Se accumulation in the shoots was greater when selenate rather than selenite was added. Furthermore, under similar concentrations of selenate and selenite in the nutrient solution, cucumber was able to accumulate more Se (over twofold) in shoots than lettuce tested in our previous study (Hawrylak-Nowak 2013).
The roots are crucial for correct plant functioning and quantification of root’s vitality is important both in studies concerning plant growth and nutrient dynamics (Stūrīte et al. 2005). To evaluate the effects of Se treatment on cucumber roots, a TTC reduction and lipid peroxidation tests were used as indicators. The reduction of TTC to a bright red, water-insoluble formazan is performed by the mitochondrial dehydrogenases. Therefore, the TTC test was regarded as an indicator of mitochondrial capacity and plant tissue viability (Mingji et al. 2009). In our study, selenate and selenite affected in different way the activity of roots. While selenate slightly modified the root TTC reduction activities, increased selenite concentrations considerably increased root activity. These data indicated that the viability of cucumber root tips was not inhibited even by highly phytotoxic selenite concentrations. However, such a large increase in the root TTC reduction under selenite exposure may suggest an upregulation of mitochondrial dehydrogenases activity which can lead to disturbances in cellular respiration. The finding of higher respiratory activity under selenite treatments is supported by an earlier study using maize (Girton 1974) and wheat (Yao et al. 2009) roots. Lyons et al. (2009) suggest that an increase in the total respiratory activity in leaves and flowers of selenite-treated Brassica rapa L. is due to an increase in cytochrome pathway capacity in mitochondria, mediated by cytochrome oxidase. In our experiment, a significant increase in the activity of roots in the presence of 6 and 10 µM of selenate also was found. Other studies using selenate have demonstrated that selenate exposure increased terminal electron transport system activity (Smrkolj et al. 2006).
The lipid peroxidation level, another parameter reflecting the root’s vitality, was also found to be significantly affected by Se compounds applied. Whereas selenate at wide range of concentrations (2–60 µM) inhibited the formation of harmful lipid peroxides, selenite was effective in this process in a narrower concentration range (2–6 µM). Moreover, selenite applied at concentrations higher than 20 µM caused a progressive increase in lipid peroxidation level. Similar properties of Se, antioxidative or prooxidative, have been observed previously, e.g., by Hartikainen et al. (2000).
The dual effect of selenate and selenite on the root activity and lipid peroxidation level may result from much higher concentration of Se in root tissues after selenite exposure comparing to selenate. The very high accumulation of Se in the roots of selenite-treated plants may lead to a complete disturbance of the proper root metabolism and, consequently, the whole plant, including mineral nutrients homeostasis.
A study on the concentrations of essential elements in plants is crucial in terms of both on the efficiency of biofortification process and its influence on the nutritive value of crops as well as eventual adverse changes in the ion balance, which may lead to the growth reduction. To our best knowledge, the influence of Se on the contents of macronutrients other than sulphur was the aim of some experiments (Wu and Huang 1992; Hawrylak-Nowak 2008; Feng et al. 2009; Kopsell et al. 2000; Matraszek and Hawrylak-Nowak 2009; Filek et al. 2010; Ramos et al. 2011; Saffaryazdi et al. 2012; Smoleń et al. 2014) but their results are sometimes ambiguous.
Our experiments revealed that under low Se dosages (≤6 µM), the concentrations of studied elements were generally maintained at the control level. The total N and Mg concentrations varied slightly under Se treatments, with the exception of decrease in N and Mg amounts only under highly phytotoxic selenite treatments. The N concentrations were not significantly affected by Se applied as selenate also in alfalfa (Owusu-Sekyere et al. 2013) and in Brassica oleracea (Kopsell et al. 2000). The Mg concentrations in maize were not significantly changed under selenite treatments (Hawrylak-Nowak 2008), and increased in response to increasing selenate concentrations in B. oleracea (Kopsell et al. 2000). In our study, the amounts of P were maintained at the control level when selenite was applied at concentrations of 2–10 µM and decreased if its concentrations rise. Interestingly, under selenate treatments an increase in P concentrations was found. This is probably the first reported incidence that selenate increased P accumulation in plants. The addition of selenite to the nutrient solution also inhibited P uptake by Chinese brake fern (Feng et al. 2009) and by lettuce (Matraszek and Hawrylak-Nowak 2009). In studies performed by Ramos et al. (2011) on lettuce, selenite treatments reduced P accumulation; whereas under selenate exposure, the contents of P remained unaffected. Conflicting results were reported by Kopsell et al. (2000), where under selenate exposure the P level decreased. The inhibition of P accumulation under selenite exposure might be derived from the competition between selenite and phosphate ions (Hopper and Parker 1999). In this study, the decline in the K levels was noted if the Se concentrations passed 6 µM and under selenite treatments this decline was higher comparing to selenate. The toxic selenite doses also reduced the K level in the shoots of maize (Hawrylak-Nowak 2008) and lettuce (Matraszek and Hawrylak-Nowak 2009). However, Kopsell et al. (2000) and Feng et al. (2009) reported increases in K concentrations with additions of selenate or selenite, respectively. Moreover, toxic selenite treatments (20–60 µM) provoked a decrease in the Ca concentration, but the exposition of plants to 40–80 µM of selenate induced higher accumulation of this macronutrient. Foliar Ca content was previously reported to be unaffected by selenate in B. oleracea (Kopsell et al. 2000). However, in maize (Hawrylak-Nowak 2008), tall fescue and white clover (Wu and Huang 1992) the Ca concentrations raised under Se exposure. The influence of Se on the S–SO4 concentrations was significant if selenate or selenite concentrations passed 6 and 10 µM, respectively. At concentrations higher than 6 µM, selenate induced a dose-dependent increase in the S–SO4 accumulation. On the other hand, in the presence of 20–30 µM of selenite, the S–SO4 level decreased, but increased in plants treated with 40–60 µM of this Se form. In general, Se at high concentrations caused elevated S–SO4 accumulation in cucumber shoots, and the impact of SeO4 − ions, as SO4 − ions analogue, was more evident. Selenium addition also increased S accumulation in shoots of rape and wheat (Filek et al. 2010) and in shoots of B. oleracea (Kopsell et al. 2000; Chang et al. 2008). The results of Ríos et al. (2008) on lettuce plants indicate that application of selenite, as opposed to selenate, did not affect the foliar S concentration. The interactions between Se and S nutrition studied in Arabidopsis thaliana imply that exogenous selenate can induce sulphate bioaccumulation in aboveground plant organs, probably by preventing a reduction in the abundance or/and activity of sulphate transporters by sulphate and its derivatives (White et al. 2004).
Conclusions
These results indicate that an application of either selenate or selenite at concentrations below the determined toxicity threshold may be potentially used for biofortification of cucumber with Se under hydroponic conditions. We imply that the high phytotoxicity of selenite is caused by very high accumulation of Se in the root system after selenite exposure, which perturbs roots activity and increases lipid peroxidation of cell membranes, impairing root metabolism and, consequently, leading to mineral homeostasis disorders. However, changes in plant macronutrient contents will be not expected when the cucumber will be biofortified with Se at concentrations not exceeding 10 µM. In addition, we observed that photosynthetic pigments contents and chlorophyll fluorescence parameters were more negatively affected by toxic selenite than selenate treatments. Therefore, we need to be aware that Se biofortification can influence the physiological processes and mineral balance of plants and thus affect their overall nutritional value. Although a considerable effect of Se enrichment on the growth and physiological parameters of cucumber was demonstrated in this work, more detailed studies are needed on the effect of Se on plants at the reproductive phase, especially in the aspects of the biofortification of this species with Se.
Author contribution statement
B. Hawrylak-Nowak designed experiment, conducted research, and wrote the paper; R. Matraszek was involved in the determination of photosynthetic pigments concentration and lipid peroxidation level; M. Pogorzelec contributed to the discussion and manuscript preparation and carried out statistical analysis. All the authors have read and approved the final manuscript.
References
Broadley MR, White PJ, Bryson RJ, Meacham MC, Bowen HC, Johnson SE, Hawkesford MJ, McGrath SP, Zhao FJ, Breward N, Harriman M, Tucker M (2006) Biofortification of UK food crops with selenium. Proc Nutr Soc 65:169–181
Chang PT, van Iersel MW, Randle WM, Sams CE (2008) Nutrient solution concentrations of Na2SeO4 affect the accumulation of sulfate and selenate in Brassica oleracea L. HortScience 43:913–918
Clemensson-Lindell A (1994) Triphenyltetrazolium chloride as an indicator of fine-root vitality and environmental stress in coniferous forest stands: applications and limitations. Plant Soil 159:297–300
Feng R, Wei C, Tu S, Wu F (2009) Effects of Se on the uptake of essential elements in Pteris vittata L. Plant Soil 325:123–132
Feng R, Wei C, Tu S (2013) The roles of selenium in protecting plants against abiotic stresses. Environ Exp Bot 87:58–68
Filek M, Zembala M, Kornaś A, Walas S, Mrowiec H, Hartikainen H (2010) The uptake and translocation of macro- and microelements in rape and wheat seedlings as affected by selenium supply level. Plant Soil 336:303–312
Funes-Collado V, Morell-Garcia A, Rubio R, López-Sánchez JF (2013) Selenium uptake by edible plants from enriched peat. Sci Hortic 164:428–433
Garcia-Bañuelos ML, Hermosillo-Cereceres MA, Sánchez E (2011) The importance of selenium biofortification in food crops. Curr Nutr Food Sci 7:181–190
Germ M, Stibilj V, Kreft I (2007) Metabolic importance of selenium for plants. Eur J Plant Sci Biotech 1:91–97
Girton RE (1974) Effects of selenite selenium on respiration in maize roots. Plant Soil 40:119–127
Hajiboland R, Amjad L (2007) Does antioxidant capacity of leaves play a role in growth response to selenium at different sulfur nutritional status? Plant Soil Environ 53:207–215
Hamilton SJ (2004) Review of selenium toxicity in the aquatic food chain. Sci Total Environ 326:1–31
Hartikainen H (2005) Biogeochemistry of selenium and its impact on food chain quality and human health. J Trace Elem Med Biol 18:309–318
Hartikainen H, Xue T, Piironen V (2000) Selenium as an anti-oxidant and pro-oxidant in ryegrass. Plant Soil 225:193–200
Hawrylak-Nowak B (2008) Effect of selenium on selected macronutrients in maize plants. J Elem 13:513–519
Hawrylak-Nowak B (2013) Comparative effects of selenite and selenate on growth and selenium accumulation in lettuce plants under hydroponic conditions. Plant Growth Reg 70:149–157
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Calif Agric Exp Sta Circ 347:1–32
Hopper JL, Parker DR (1999) Plant availability of selenite and selenate as influenced by the competing ions phosphate and sulfate. Plant Soil 210:199–207
Kopsell DA, Kopsell DE (2007) Selenium. In: Barker AV, Pilbeam DJ (eds) Handbook of plant nutrition. CRC Press, Boca Raton, pp 515–549
Kopsell DA, Randle WM, Mills HA (2000) Nutrient accumulation in leaf tissue of rapid-cycling Brassica oleracea responds to increasing sodium selenate concentrations. J Plant Nutr 23:927–935
Lichtenthaler HK, Wellburn AR (1983) Determination of total carotenoids and chlorophyll a and b of leaf extracts in different solvents. Biochem Soc Trans 603:591–592
Lyons G, Genc Y, Soole K, Stangoulis JC, Liu F, Graham RD (2009) Selenium increases seed production in Brassica. Plant Soil 318:73–80
Matraszek R, Hawrylak-Nowak B (2009) Macronutrients accumulation in useable parts of lettuce as affected by nickel and selenium concentrations in nutrient solution. Fresenius Environ Bull 18:1059–1065
Mingji X, Chongling Y, Jing Y, Lily W (2009) Impact of phenanthrene on organic acids secretion and accumulation by perennial ryegrass, Lolium perenne L., root. Bull Environ Contam Toxicol 83:75–80
Nowosielski O (1974) Methods for the determination of fertilisation requirements. PWRiL, Warszawa (in Polish)
Owusu-Sekyere A, Kontturi J, Hajiboland R, Rahmat S, Aliasgharzad N, Hartikainen H, Seppänen MM (2013) Influence of selenium (Se) on carbohydrate metabolism, nodulation and growth in alfalfa (Medicago sativa L.). Plant Soil 373:541–552
Ramos SJ, Faquin V, de Almeida HJ, Ávila FW, Guimarães Guilherme LR, Alves Bastos CE, Ávila PA (2011) Selenate and selenite on yield, mineral nutrition and biofortification with selenium in lettuce cultivars. Rev Bras Cienc Solo 35:1347–1355
Ríos JJ, Blasco B, Cervilla LM, Rubio-Wilhelmi MM, Ruiz JM, Romero L (2008) Regulation of sulphur assimilation in lettuce plants in the presence of selenium. Plant Growth Reg 56:43–51
Saffaryazdi A, Lahouti M, Ganjeali A, Bayat H (2012) Impact of selenium supplementation on growth and selenium accumulation on spinach (Spinacia oleracea L.) plants. Not Sci Biol 4:95–100
Schreiber U, Bilger W, Neubauer C (1994) Chlorophyll fluorescence as a non-destructive indicator for rapid assessment of in vivo photosynthesis. Ecol Stud 100:49–70
Smoleń S, Kowalska I, Sady W (2014) Assessment of biofortification with iodine and selenium of lettuce cultivated in the NFT hydroponic system. Sci Hortic 166:9–16
Smrkolj P, Germ M, Kreft I, Stibilj V (2006) Respiratory potential and Se compounds in pea (Pisum sativum L.) plants grown from Se-enriched seeds. J Exp Bot 57:3595–3600
Stūrīte I, Henriksen TM, Breland TA (2005) Distinguishing between metabolically active and inactive roots by combined staining with 2,3,5-triphenyltetrazolium chloride and image colour analysis. Plant Soil 271:75–82
Terry N, Zayed AM, De Souza MP, Tarun AS (2000) Selenium in higher plants. Ann Rev Plant Physiol Plant Mol Biol 51:401–432
Valdez Barillas JR, Quin CF, Pilon-Smits EAH (2011) Selenium accumulation in plants—phytotechnological applications and ecological implications. Int J Phytorem 13:166–178
Valkama E, Kivimäenpää M, Hartikainen H, Wulff A (2003) The combined effects of enhanced UV-B radiation and selenium on growth, chlorophyll fluorescence and ultrastructure in strawberry (Fragaria × ananassa) and barley (Hordeum vulgare) treated in the field. Agric For Meteor 120:267–278
White PJ, Broadley MR (2009) Biofortification of crops with seven mineral elements often lacking in human diets – iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol 182:49–84
White PJ, Bowen HC, Parmaguru P, Fritz M, Spracklen WP, Spiby RE, Meacham MC, Mead A, Harriman M, Trueman LJ, Smith BM, Thomas B, Broadley MR (2004) Interactions between selenium and sulphur nutrition in Arabidopsis thaliana. J Exp Bot 55:1927–1937
Wu L, Huang ZZ (1992) Selenium assimilation and nutrient element uptake in white clover and tall fescue under the influence of sulphate concentration and selenium tolerance of the plants. J Exp Bot 43:549–555
Yao X, Chu J, Wang G (2009) Effects of drought stress and selenium supply on growth and physiological characteristics of wheat seedlings. Acta Physiol Plant 31:1031–1036
Zayed A, Lytle CM, Terry N (1998) Accumulation and volatilization of different chemical species of selenium by plants. Planta 206:284–292
Zhang M, Tang S, Huang X, Zhang F, Pang Y, Huang Q, Yi Q (2014) Selenium uptake, dynamic changes in selenium content and its influence on photosynthesis and chlorophyll fluorescence in rice (Oryza sativa L.). Environ Exp Bot 107:39–45
Acknowledgments
This research was financially supported by the Grant No. N N310 430939 of the Polish Ministry of Science and Higher Education.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Kovacik.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Hawrylak-Nowak, B., Matraszek, R. & Pogorzelec, M. The dual effects of two inorganic selenium forms on the growth, selected physiological parameters and macronutrients accumulation in cucumber plants. Acta Physiol Plant 37, 41 (2015). https://doi.org/10.1007/s11738-015-1788-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-015-1788-9