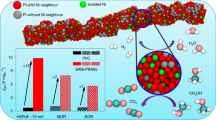
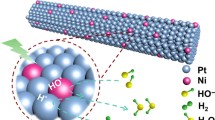
Abstract
In this paper, ultrathin Pt nanowires (Pt NWs) and PtNi alloy nanowires (PtNi NWs) supported on carbon were synthesized as electrocatalysts for oxygen reduction reaction (ORR). Pt and PtNi NWs catalysts composed of interconnected nanoparticles were prepared by using a soft template method with CTAB as the surface active agent. The physical characterization and electrocatalytic performance of Pt NWs and PtNi NWs catalysts for ORR were investigated and the results were compared with the commercial Pt/C catalyst. The atomic ratio of Pt and Ni in PtNi alloy was approximately 3 to 1. The results show that after alloying with Ni, the binding energy of Pt shifts to higher values, indicating the change of its electronic structure, and that Pt3Ni NWs catalyst has a significantly higher electrocatalytic activity and good stability for ORR as compared to Pt NWs and even Pt/C catalyst. The enhanced electrocatalytic activity of Pt3Ni NWs catalyst is mainly resulted from the downshifted-band center of Pt caused by the interaction between Pt and Ni in the alloy, which facilitates the desorption of oxygen containing species (Oads or OHads) and the release of active sites.
Similar content being viewed by others
References
Debe M K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature, 2012, 486(7401): 43–51
Tang Q, Jiang L, Jiang Q, Wang S, Sun G. Enhanced activity and stability of a Au decorated Pt/PdCo/C electrocatalyst toward oxygen reduction reaction. Electrochimica Acta, 2012, 77(9): 104–110
Bele M, Jovanovic P, Pavlisic A, Jozinovic B, Zorko M, Recnik A, Chernyshova E, Hocevar S, Hodnik N, Gaberscek M. A highly active PtCu3 intermetallic core-shell, multilayered Pt-skin, carbon embedded electrocatalyst produced by a scale-up sol-gel synthesis. Chemical Communications, 2014, 50(86): 13124–13126
Zhang J, Sasaki K, Sutter E, Adzic R R. Stabilization of platinum oxygen-reduction electrocatalysts using gold clusters. Science, 2007, 315(5809): 220–222
Wang Y J, Zhao N N, Fang B Z, Li H, Bi X T T,Wang H J. Carbonsupported Pt-based alloy electrocatalysts for the oxygen reduction reaction in polymer electrolyte membrane fuel cells: particle size, shape, and composition manipulation and their impact to activity. Chemical Reviews, 2015, 115(9): 3433–3467
Chen Z W, Higgins D, Yu A P, Zhang L, Zhang J J. A review on non-precious metal electrocatalysts for PEM fuel cells. Energy & Environmental Science, 2011, 4(9): 3167–3192
Zheng Y, Jiao Y, Jaroniec M, Jin Y G, Qiao S Z. Nanostructured metal-free electrochemical catalysts for highly efficient oxygen reduction. Small, 2012, 8(23): 3550–3566
Bai Y Z, Yi B L, Li J, Jiang S F, Zhang H J, Shao Z G, Song Y J. A high performance non-noble metal electrocatalyst for the oxygen reduction reaction derived from a metal organic framework. Chinese Journal of Catalysis, 2016, 37(7): 1127–1133
Greeley J, Mavrikakis M. Alloy catalysts designed from first principles. Nature Materials, 2004, 3(11): 810–815
Wu J B, Yang H. Platinum-based oxygen reduction electrocatalysts. Accounts of Chemical Research, 2013, 46(8): 1848–1857
Zhang J, Mo Y, Vukmirovic M B, Klie R, Sasaki K, Adzic R R. Platinum monolayer electrocatalysts for O2 reduction: Pt monolayer on Pd(111) and on carbon-supported Pd nanoparticles. Journal of Physical Chemistry B Materials Surfaces Interfaces Amp Biophysical, 2004, 108(30): 10955–10964
Zhang J L, Vukmirovic M B, Xu Y, Mavrikakis M, Adzic R R. Controlling the catalytic activity of platinum-monolayer electrocatalysts for oxygen reduction with different substrates. Angewandte Chemie, 2005, 44(14): 2132–2135
Zhu H Y, Zhang S, Guo S J, Su D, Sun S H. Synthetic control of FePtM nanorods (M = Cu, Ni) to enhance the oxygen reduction reaction. Journal of the American Chemical Society, 2013, 135(19): 7130–7133
You H J, Yang S C, Ding B J, Yang H. Synthesis of colloidal metal and metal alloy nanoparticles for electrochemical energy applications. ChemInform, 2013, 42(7): 2880–2904
Zhao X, Yin M, Ma L, Liang L, Liu C P, Liao J H, Lu T H, Xing W. Recent advances in catalysts for direct methanol fuel cells. Energy & Environmental Science, 2011, 4(8): 2736–2753
Li Y J, Chen L, Chen K, Quan F X, Chen C F. Monodisperse PdCu@PtCu Core@Shell nanocrystal and their high activity and durability for oxygen reduction reaction. Electrochimica Acta, 2016, 192: 227–233
Wang G, Huang B, Xiao L, Ren Z, Chen H, Wang D, Abruña H D, Lu J, Zhuang L. Pt skin on AuCu intermetallic substrate: a strategy to maximize Pt utilization for fuel cells. Journal of the American Chemical Society, 2014, 136(27): 9643–9649
Huang X Q, Zhao Z P, Chen Y, Zhu E B, Li M F, Duan X F, Huang Y. A rational design of carbon-supported dispersive Pt-based octahedra as efficient oxygen reduction reaction catalysts. Energy & Environmental Science, 2014, 7(9): 2957–2962
Huang X Q, Zhao Z P, Cao L, Chen Y, Zhu E B, Lin Z Y, Li M F, Yan A M, Zettl A, Wang Y M, Duan X F, Mueller T, Huang Y. High-performance transition metal-doped Pt3Ni octahedra for oxygen reduction reaction. Science, 2015, 348(6240): 1230–1234
Chen C, Kang Y J, Huo Z Y, Zhu Z W, Huang W Y, Xin H L L, Snyder J D, Li D G, Herron J A, Mavrikakis M, Chi M F, More K L, Li Y D, Markovic N M, Somorjai G A, Yang P D, Stamenkovic V R. Highly crystalline multimetallic nanoframes with three-dimensional electrocatalytic surfaces. Science, 2014, 343(6177): 1339–1343
Yang H Z, Zhang J, Sun K, Zou S Z, Fang J Y. Enhancing by weakening: electrooxidation of methanol on Pt3Co and Pt nanocubes. Angewandte Chemie International Edition in English, 2010, 49(38): 6848–6851
Wang S Y, Jiang S P, Wang X, Guo J. Enhanced electrochemical activity of Pt nanowire network electrocatalysts for methanol oxidation reaction of fuel cells. Electrochimica Acta, 2011, 56(3): 1563–1569
Pozio A, de Francesco M, Cemmi A, Cardellini F, Giorgi L. Comparison of high surface Pt/C catalysts by cyclic voltammetry. Journal of Power Sources, 2002, 105(1): 13–19
Stamenkovic V R, Fowler B, Mun B S, Wang G F, Ross P N, Lucas C A, Markovic N M. Improved oxygen reduction activity on Pt3Ni (111) via increased surface site availability. Science, 2007, 315 (5811): 493–497
Bu L Z, Zhang N, Guo S J, Zhang X, Li J, Yao J L, Wu T, Lu G, Ma J Y, Su D, Huang X Q. Biaxially strained PtPb/Pt core/shell nanoplate boosts oxygen reduction catalysis. Science, 2016, 354 (6318): 1410–1414
Suo Y G, Zhuang L, Lu J T. First-principles considerations in the design of Pd-alloy catalysts for oxygen reduction. Angewandte Chemie, 2007, 46(16): 2862–2864
Jayasayee K, van Veen J A R, Manivasagam T G, Celebi S, Hensen E J M, de Bruijn F A. Oxygen reduction reaction (ORR) activity and durability of carbon supported PtM (Co, Ni, Cu) alloys: influence of particle size and non-noble metals. Applied Catalysis B: Environmental, 2012, 111–112(2): 515–526
Wang D S, Li Y D. Bimetallic nanocrystals: liquid-phase synthesis and catalytic applications. Advanced Materials, 2011, 23(9): 1044–1060
Acknowledgements
This work is financially supported by The National Key Research and Development Program of China (Grant No. 2016YFB0101208) and National Natural Science Foundation of China (Grant No.U1508202 and No.61433013).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, H., Zeng, Y., Cao, L. et al. Enhanced electrocatalytic performance of ultrathin PtNi alloy nanowires for oxygen reduction reaction. Front. Energy 11, 260–267 (2017). https://doi.org/10.1007/s11708-017-0499-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11708-017-0499-x