Abstract
Background
Recent studies have suggested that obesity is associated with an increased intestinal permeability as well as an altered microbiota profile. These conditions can promote the translocation of lipopolysaccharide into the circulation and, subsequently, contribute to the observed systemic inflammation. Our aim was to assess gut permeability in patients with obesity compared to non-obese subjects as well as after excessive weight loss following laparoscopic sleeve gastrectomy (LSG).
Methods
We analyzed the dietary intake, metabolic and inflammatory markers, gut permeability (four-probe sugar test), and microbiota composition in 17 morbidly obese patients before and after LSG as well as in 17 age- and gender-matched non-obese subjects. Additionally, we compared gut permeability and inflammatory markers in patients of different stages of obesity.
Results
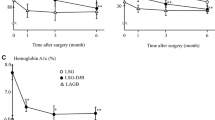
Patients with obesity showed elevated levels of C-reactive protein and lipopolysaccharide-binding protein as compared to non-obese subjects, but no differences were noted for gut permeability between these two groups. LSG led to improvements in metabolic and inflammatory parameters in the obese patients. Moreover, gastroduodenal as well as small intestinal permeability decreased, whereas colonic permeability increased after surgery. Regarding gut microbiota, differences were noted for main phyla and alpha-diversity between non-obese and obese subjects. After surgery, the composition of the microbiota showed a tendency toward the pattern of the non-obese group.
Conclusions
Gut permeability is not dependent on body mass index, whereas weight loss after LSG initiates distinct changes in gastroduodenal, intestinal, and colonic permeability. These changes do not seem to be associated with changes in the microbiota composition.
Clinical Trial Registry Number and Website
The trials were registered at https://www.drks.de/drks_web/ with the number DRKS00009008 and DRKS00006210.






Similar content being viewed by others
Change history
29 April 2021
A Correction to this paper has been published: https://doi.org/10.1007/s11695-021-05439-1
References
Han TS, Lean ME. A clinical perspective of obesity, metabolic syndrome and cardiovascular disease. JRSM Cardiovasc Dis. 2016;5:1–13. https://doi.org/10.1177/2048004016633371.
Nguyen NT, Varela JE. Bariatric surgery for obesity and metabolic disorders: state of the art. Nat Rev Gastroenterol Hepatol. 2017;14:160–9. https://doi.org/10.1038/nrgastro.2016.170.
Cani PD, Neyrinck AM, Fava F, et al. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50:2374–83. https://doi.org/10.1007/s00125-007-0791-0.
Cani PD, Bibiloni R, Knauf C, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–81. https://doi.org/10.2337/db07-1403.
Lam YY, Ha CWY, Campbell CR, et al. Increased gut permeability and microbiota change associate with mesenteric fat inflammation and metabolic dysfunction in diet-induced obese mice. PLoS One. 2012;7:e34233. https://doi.org/10.1371/journal.pone.0034233.
Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–72. https://doi.org/10.2337/db06-1491.
Verdam FJ, Fuentes S, de JC, et al. Human intestinal microbiota composition is associated with local and systemic inflammation in obesity. Obesity (Silver Spring). 2013;21:E607–15. https://doi.org/10.1002/oby.20466.
Teixeira TFS, Souza NCS, Chiarello PG, et al. Intestinal permeability parameters in obese patients are correlated with metabolic syndrome risk factors. Clin Nutr. 2012;31:735–40. https://doi.org/10.1016/j.clnu.2012.02.009.
Gummesson A, Carlsson LMS, Storlien LH, et al. Intestinal permeability is associated with visceral adiposity in healthy women. Obesity (Silver Spring). 2011;19:2280–2. https://doi.org/10.1038/oby.2011.251.
Brignardello J, Morales P, Diaz E, et al. Pilot study: alterations of intestinal microbiota in obese humans are not associated with colonic inflammation or disturbances of barrier function. Aliment Pharmacol Ther. 2010;32:1307–14. https://doi.org/10.1111/j.1365-2036.2010.04475.x.
Savassi-Rocha AL, Diniz MTC, Vilela EG, et al. Changes in intestinal permeability after Roux-en-Y gastric bypass. Obes Surg. 2014;24:184–90. https://doi.org/10.1007/s11695-013-1084-y.
Blanchard C, Moreau F, Chevalier J, et al. Sleeve gastrectomy alters intestinal permeability in diet-induced obese mice. Obes Surg. 2017;27:2590–8. https://doi.org/10.1007/s11695-017-2670-1.
Ott B, Skurk T, Hastreiter L, et al. Effect of caloric restriction on gut permeability, inflammation markers, and fecal microbiota in obese women. Sci Rep. 2017;7:11955. https://doi.org/10.1038/s41598-017-12109-9.
Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9.
Norman K, Pirlich M, Schulzke J-D, et al. Increased intestinal permeability in malnourished patients with liver cirrhosis. Eur J Clin Nutr. 2012;66:1116–9. https://doi.org/10.1038/ejcn.2012.104.
Lagkouvardos I, Kläring K, Heinzmann SS, et al. Gut metabolites and bacterial community networks during a pilot intervention study with flaxseeds in healthy adult men. Mol Nutr Food Res. 2015;59:1614–28. https://doi.org/10.1002/mnfr.201500125.
Lagkouvardos I, Joseph D, Kapfhammer M, et al. IMNGS: a comprehensive open resource of processed 16S rRNA microbial profiles for ecology and diversity studies. Sci Rep. 2016;6:33721. https://doi.org/10.1038/srep33721.
Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods. 2013;10:996–8. https://doi.org/10.1038/nmeth.2604.
Jackson MA, Goodrich JK, Maxan M-E, et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut. 2016;65:749–56. https://doi.org/10.1136/gutjnl-2015-310861.
Imhann F, Bonder MJ, Vila AV, et al. Proton pump inhibitors affect the gut microbiome. Gut. 2016;65:740–8. https://doi.org/10.1136/gutjnl-2015-310376.
Hoogerboord M, Wiebe S, Klassen D, et al. Laparoscopic sleeve gastrectomy: perioperative outcomes, weight loss and impact on type 2 diabetes mellitus over 2 years. Can J Surg. 2014;57:101–5.
Roa PE, Kaidar-Person O, Pinto D, et al. Laparoscopic sleeve gastrectomy as treatment for morbid besity: technique and short-term outcome. Obes Surg. 2006;16:1323–6.
Fasano A, Fiorentini C, Donelli G, et al. Zonula occludens toxin modulates tight junctions through protein kinase C-dependent actin reorganization, in vitro. J Clin Invest. 1995;96:710–20. https://doi.org/10.1172/JCI118114.
Teixeira TFS, Collado MC, Ferreira CLLF, et al. Potential mechanisms for the emerging link between obesity and increased intestinal permeability. Nutr Res. 2012;32:637–47. https://doi.org/10.1016/j.nutres.2012.07.003.
Scheffler L, Crane A, Heyne H, et al. Widely used commercial ELISA does not detect precursor of haptoglobin2, but recognizes properdin as a potential second member of the zonulin family. Front Endocrinol (Lausanne). 2018;9:22. https://doi.org/10.3389/fendo.2018.00022.
Shah S, Shah P, Todkar J, et al. Prospective controlled study of effect of laparoscopic sleeve gastrectomy on small bowel transit time and gastric emptying half-time in morbidly obese patients with type 2 diabetes mellitus. Surg Obes Relat Dis. 2010;6:152–7. https://doi.org/10.1016/j.soard.2009.11.019.
Tuomi K, Logomarsino JV. Bacterial lipopolysaccharide, lipopolysaccharide-binding protein, and other inflammatory markers in obesity and after bariatric surgery. Metab Syndr Relat Disord. 2016;14:279–88. https://doi.org/10.1089/met.2015.0170.
van Dielen FMH, Buurman WA, Hadfoune M, et al. Macrophage inhibitory factor, plasminogen activator inhibitor-1, other acute phase proteins, and inflammatory mediators normalize as a result of weight loss in morbidly obese subjects treated with gastric restrictive surgery. J Clin Endocrinol Metab. 2004;89:4062–8. https://doi.org/10.1210/jc.2003-032125.
Moschen AR, Molnar C, Geiger S, et al. Anti-inflammatory effects of excessive weight loss: potent suppression of adipose interleukin 6 and tumour necrosis factor alpha expression. Gut. 2010;59:1259–64. https://doi.org/10.1136/gut.2010.214577.
Skurk T, Alberti-Huber C, Herder C, et al. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab. 2007;92:1023–33. https://doi.org/10.1210/jc.2006-1055.
Damms-Machado A, Louis S, Schnitzer A, et al. Gut permeability is related to body weight, fatty liver disease, and insulin resistance in obese individuals undergoing weight reduction. Am J Clin Nutr. 2017;105:127–35. https://doi.org/10.3945/ajcn.116.131110.
Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–4. https://doi.org/10.1038/nature07540.
Ley RE, Bäckhed F, Turnbaugh P, et al. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102:11070–5. https://doi.org/10.1073/pnas.0504978102.
Arumugam M, Raes J, Pelletier E, et al. Enterotypes of the human gut microbiome. Nature. 2011;473:174–80. https://doi.org/10.1038/nature09944.
Costea PI, Hildebrand F, Arumugam M, et al. Enterotypes in the landscape of gut microbial community composition. Nat Microbiol. 2018;3:8–16. https://doi.org/10.1038/s41564-017-0072-8.
Murphy R, Tsai P, Jüllig M, et al. Differential changes in gut microbiota after gastric bypass and sleeve gastrectomy bariatric surgery vary according to diabetes remission. Obes Surg. 2017;27:917–25. https://doi.org/10.1007/s11695-016-2399-2.
Damms-Machado A, Mitra S, Schollenberger AE, et al. Effects of surgical and dietary weight loss therapy for obesity on gut microbiota composition and nutrient absorption. Biomed Res Int. 2015;2015:806248–12. https://doi.org/10.1155/2015/806248.
Tremaroli V, Karlsson F, Werling M, et al. Roux-en-Y gastric bypass and vertical banded gastroplasty induce long-term changes on the human gut microbiome contributing to fat mass regulation. Cell Metab. 2015;22:228–38. https://doi.org/10.1016/j.cmet.2015.07.009.
Patil DP, Dhotre DP, Chavan SG, et al. Molecular analysis of gut microbiota in obesity among Indian individuals. J Biosci. 2012;37:647–57. https://doi.org/10.1007/s12038-012-9244-0.
Acknowledgments
We highly acknowledge Prof. Dr. Thomas Hüttl, Dr. Peter Stauch, Dr. Otto Dietl, and Hildegard Wood together with their whole team at the Adipositas Zentrum München Bogenhausen; Dr. Georg Dechantsreiter and team at the Krankenhaus Landshut Achdorf; and Dr. Andreas Limberger and team at the Kreiskrankenhaus Schrobenhausen for their support in recruitment and sample collection. Additionally, we are thankful to Martina Werich for the analysis of sugars in the urine samples. We are grateful to Manuela Hubersberger for her excellent technical assistance and to the ZIEL Core Facility Microbiome/NGS at the TU Munich for support with high-throughput 16S rRNA gene amplicon sequencing and analysis.
Funding
The study was founded by the DFG (Deutsche Forschungsgemeinschaft) as part of the Graduiertenkolleg 1482 as well as by the BMBF (Federal Ministry of Education and Research, grant no. 0315674) and in part by the Else Kröner-Fresenius-Foundation, Bad Homburg v. d. H., Germany.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Beate Brandl Née: Ott
Electronic Supplementary Material
Supplemental Figure 1
Multidimensional scaling (MDS) plot of phylogenetic distances (β-diversity). Non-obese: non-obese group, n = 17; PPI V1: obese patients with PPI medication before surgery, n = 5; no PPI V1: obese patients without PPI medication before surgery, n = 12; PPI V2: obese patients with PPI medication 6 months after surgery, n = 7; no PPI V2: obese patients without PPI medication 6 months after surgery, n = 10. (PNG 356 kb)
High resolution image
(EPS 968 kb)
ESM 2
(DOCX 23.0 kb)
ESM 3
(DOCX 26.6 kb)
ESM 4
(DOCX 28.5 kb)
ESM 5
(DOCX 22.5 kb)
Rights and permissions
About this article
Cite this article
Kellerer, T., Brandl, B., Büttner, J. et al. Impact of Laparoscopic Sleeve Gastrectomy on Gut Permeability in Morbidly Obese Subjects. OBES SURG 29, 2132–2143 (2019). https://doi.org/10.1007/s11695-019-03815-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-019-03815-6