Abstract
Members of the mammalian families Elephantidae and Hippopotamidae (extant and extinct elephants and hippos) include extinct dwarf species that display up to 98% decrease in body size compared to probable ancestral sources. In addition to differences in body mass, skulls of these species consistently display distinctive morphological changes, including major reduction of pneumatised areas in dwarf elephants and shortened muzzles in dwarf hippos. Here we build on previous studies of island dwarf species by conducting a geometric morphometric analysis of skull morphology and allometry in target taxa, living and extinct, and elaborate on the relation between skull size and body size. Our analysis indicates that skull size and body size within terrestrial placental mammals scale almost isometrically (PGLS major axis slope 0.906). Furthermore, skull shape in dwarf species differed from both their ancestors and the juveniles of extant species. In insular dwarf hippos, the skull was subject to considerable anatomical reorganisation in response to distinct selection pressures affecting early ontogeny (the “island syndrome”). By contrast, skull shape in adult insular dwarf elephants can be explained well by allometric effects; selection on size may thus have been the main driver of skull shape in dwarf elephants. We suggest that a tightly constrained growth trajectory, without major anatomical reorganization of the skull, allowed for flexible adaptations to changing environments and was one of the factors underlying the evolutionary success of insular dwarf elephants.
Similar content being viewed by others
Introduction
The paleontological record suggests that propagules of megafaunal mammal species that successfully migrated to islands tended to lose genetic contact with their mainland relatives. One of the most obvious outcomes of such isolation and associated new selective pressure was that insular populations often evolved smaller body sizes, leading to such bizarre-looking animals as pony-sized elephants and pig-sized hippos (Sondaar 1994). This pattern of anagenetic, or phyletic, dwarfing under insular conditions, generally described in terms of the ‘island rule’ (Van Valen 1973 and subsequent authors), is pervasive and is exhibited by extant as well as extinct species (Lomolino et al. 2013). The speed of phyletic dwarfing varies, but it may occur at extremely fast rates (Lister 1989; Pergams and Ashley 1999; Millien 2006; Evans et al. 2012; van den Bergh et al. 2016). There is an extensive literature on the biotic and abiotic conditions that appear to underlie insular phyletic dwarfing (see Lomolino et al. 2012, 2013 for a synthesis). Equally extensive is the morphological literature on the dwarfs themselves, which is generally aimed at systematic resolution of their affinities (see Van der Geer et al. 2010 and Van der Geer 2014 for an overview). However, literature on the ontogenetic mechanisms underlying phyletic dwarfing is much sparser.
Sondaar (1977) speculated that cranial differences between the Sicilian dwarf elephant Palaeoloxodon falconeri and its mainland relative Palaeoloxodon antiquus resulted from allometric effects, such as a decrease in muscle attachment areas due a lighter skull and smaller tusks. Roth (1984) was the first to thoroughly investigate the mechanism of phyletic dwarfing in fossil elephants. She argued that the dwarfing process is complex and that the large intraspecific variation in size and morphology seen in many dwarfed taxa may reflect a range of mechanisms involved (Roth 1984). In her view, the substantial morphological variability found within dwarf elephant populations suggests that small body size, rather than explicitly pedomorphic morphology, was the target of selection (Roth 1984, 1993). Elsewhere, however, she pointed to presumably pedomorphic features in dwarf elephant skulls (Roth 1992) relative to the postcranium, where she had earlier found peramorphic features (Roth 1984). Lister (1989) graphically illustrated the similarity between the skull of P. falconeri and that of very young extant elephants, but pointed out that the presence of large tusks cannot be considered a pedomorphic trait. Later, Lister (1996) concluded that the early stages of dwarfism in both insular elephants and deer lead to suboptimal results, due to the characteristically (very) short interval between propagule arrival and the establishment of a dwarfing lineage. After an initial rapid stage of downsizing, a prolonged period of slow but extensive modification would ensue, eventually resulting in significant size change (Lister 1996). In regard to cranial changes, Lister (1996) specifically noted that species that have been isolated for geologically short periods of time have relatively large teeth, whereas this is not the case after extended periods of isolation.
In this contribution, our aim is to test earlier claims that the skulls of insular dwarf forms are pedomorphic (e.g. Lister 1989; Palombo 2001; Van der Geer 2005; Van Heteren 2008; Masters et al. 2014; see also above). To this end, we compare the morphological consequences of phyletic dwarfing in fossil elephants and hippos to ontogenetic shape changes in extant representatives of these groups using geometric morphometrics, which allows for explicit estimates of size and shape parameters (Penin et al. 2002; Mitteroecker et al. 2005, 2013). This methodology has been used to successfully characterize phyletic dwarfing in extant taxa (e.g., in bovids, Chiozzi et al. 2014; in lemurs, Masters et al. 2014). However, this approach has not been applied to taxa that have experienced extreme size reduction of the sort discussed here. In some instances, body sizes in insular elephants and hippos were no more than 2–4% of ancestral mainland size (Lomolino et al. 2013)—the greatest reduction within single lineages recorded among mammals. Such a scale of change provides an unprecedented opportunity to investigate evolutionary processes involved in dwarfing.
Our targets are straight-tusked elephants of the genus Palaeoloxodon and the hippopotamid Hippopotamus. In the case of the elephantids we focus on comparing overall size reduction from the probable ancestral form, mainland P. antiquus, and P. ‘mnaidriensis’ (late Pleistocene of Sicily; with 83% body mass reduction) and P. falconeri (middle Pleistocene of Sicily; a truly remarkable 98% reduction) (Fig. 1). With regard to hippos, we quantify cranial differences between mainland Hippopotamus amphibius and the extinct Quaternary Malagasy taxa H. madagascariensis and H. lemerlei, which experienced 75% body mass reduction. We also investigate differences between mainland H. amphibius and H. minor (late Pleistocene of Cyprus; 96% reduction in body mass). Our sample includes dwarfs of different sizes, which enables us to model morphological changes at different degrees of phyletic dwarfing where temporal series from a single island are lacking.
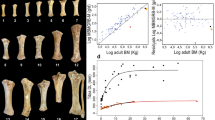
Skeletal mounts of the species involved in this study. Note that the skeletons of the mainland species belong to single individuals, while those of the insular species are composite mounts. Palaeoloxodon antiquus (Museo di Paleontologìa, Università di Roma 'La Sapienza', Rome, Italy), P. ‘mnaidriensis’ (MGG), P. falconeri (SFN, cast of original in Museo di Paleontologìa, Rome, Italy), Hippopotamus antiquus (Museo di Storia Naturale, Geologia e Paleontologia, Università di Firenze, Florence, Italy), H. amphibius (Universiteitsmuseum Utrecht, the Netherlands), H. minor (AMPG), H. lemerlei (MFN). For institutional abbreviations see Table 1
Materials and Methods
Taxonomic Framework
We follow Boisserie (2005) in considering the mainland pygmy hippopotamus of West Africa as a member of the genus Choeropsis. We follow Stuenes (1989) in referring the Malagasy dwarf hippos to Hippopotamus. Although we are aware of the fact that the species names H. lemerlei and H. madagascariensis should be taxonomically revised (Goodman and Jungers 2014), and that the latter species is sometimes referred to as H. guldbergi (Fovet et al. 2011), pending better systematic resolution of these issues we will use species names and limits as defined by Stuenes (1989). The Cypriot dwarf hippo is often placed in a genus of its own (Phanourios, described by Boekschoten and Sondaar 1972). However, since it descended directly from Hippopotamus (Boekschoten and Sondaar 1972; Houtekamer and Sondaar 1979; Boisserie 2005), we here treat it as a member of Hippopotamus, following Van der Geer et al. (2010).
Genetic evidence (Roca et al. 2001; Palkopoulou et al. 2018) has confirmed the independent taxonomic status of the African forest elephant, Loxodonta cyclotis, which we follow here. Palaeoloxodon mnaidriensis is a dwarf elephant species of Malta. The same name has been extensively used for the larger dwarf elephant of Sicily. However, the specific status of the Sicilian material (Puntali Cave) has been questioned (Ferretti 2008; Herridge 2010) and probably a new species should be erected to accommodate the Sicilian material (Palaeoloxodon sp. nov. in Herridge 2010). For that reason, we list the Sicilian material here as P. ‘mnaidriensis’, following Van der Geer et al. (2014).
Phylogeny
It is important to note here that the focal species do not represent stages of an actual evolutionary sequence. The two Sicilian dwarf elephants (P. ‘mnaidriensis’ and P. falconeri) resulted from two independent colonisations (Ferretti 2008). In the past, the occurrence of the two differently sized dwarf species was explained by anagenesis, with the smallest species having evolved from the larger species. The deposits with the larger species, however, appeared to be late Pleistocene in age, whereas those with the smaller species are attributed to the middle Pleistocene (for a historical overview, see Van der Geer et al. 2010). Contrary to the Sicilian dwarf elephants, the two Malagasy hippopotamuses (H. madagascariensis and H. lemerlei) are probably the result of a single colonization followed by in situ speciation.
Obviously, it would have been ideal to utilize cranial material from single lineages, from initial colonization by the mainland ancestral species, through stages of insular adaptation, to the ultimate result, the endemic dwarfs. The reality is that time series are extremely rare in the insular fossil record in general (Lyras et al. 2010; Van der Geer et al. 2013); for elephants there is very little (van den Bergh et al. 2009 for Flores; Athanassiou et al. 2015 for Cyprus) and for hippos there is nothing at all. We know that size reduction occurred extremely quickly in some island contexts (e.g., red deer on the Channel Islands; Lister 1989), which may help to explain the lack of intermediates. In any case, fairly complete skulls are very rare in general, especially so for elephants, and unknown for any single insular lineage. Therefore, our necessarily polyphyletic approach is the only practical way to investigate cranial changes that took place during island dwarfing in elephant and hippo lineages. Nevertheless, the species selected for analysis are phylogenetically very close, which allows us to reasonably treat them as model stages illustrating different degrees of phyletic dwarfing.
Age Determination of Hippopotamuses and Elephants
The chronological ages of individual fossil elephants and hippos cannot be estimated directly, because no data exist on age-related changes in the dentitions of the focal insular species. As a proxy we have utilized currently-available dental criteria for determining chronological age in extant elephants and hippos. These methods are, however, based on lower dentitions (Laws 1966, 1968; Roth and Shoshani 1988). Many skulls in zoological and paleontological collections lack associated mandibles, but rather than reject this material as unusable we applied the same criteria to upper dentitions in order to assess age. There is no reason to expect this to have biased our results in a single direction. In taking this approach we follow Weston (1998) and Weston and Lister (2009) with respect to fossil hippos.
Individual age estimation of hippos is based on papers by Laws (1968) for Hippopotamus and Weston (1998) for Choeropsis. Hippos are here considered adult when their third lower molar is completely erupted (Laws’ stage XIII, here 13), which is around 24–27 years in H. amphibius and 8–10 years in C. liberiensis. However, sexual maturity is achieved much earlier (7.5–9 years in H. amphibius and 3–4 years in C. liberiensis; Weston 1998).
Age estimation of elephants is based on dental eruption and wear patterns following Roth (1984) and Roth and Shoshani (1988) for Elephas maximus and Laws (1966) for Loxodonta africana and L. cyclotis. The distinction between calves/juveniles versus subadults/adults follows Sikes (1967). Skeletal fusion, an indication of skeletal maturity, starts at dental stages 11 (L. africana) or 12 (E. maximus) (Roth 1984).
Although chronological age can also be determined using incremental growth lines in long bones (e.g. Kolb et al. 2015), dental hard tissues (e.g. Iinuma et al. 2004), and dental cementum (e.g. Kubo et al. 2011), the methods require destructive sampling and we had insufficient material for this purpose.
Material
Geometric morphometric data on fossil skulls (see Table 1 for list of specimens) were collected for six elephants and eight hippos. Our extant comparative sample (see Table 2 for list of specimens) comprises 30 elephants and 36 hippos.
The skull of the female P. falconeri is nearly complete, while that of the male is a composite of two incomplete and partially reconstructed skulls (numbers five and six of Ambrosetti 1968). Even though the reconstruction was done with great care (Ambrosetti 1968), the position of this particular specimen in the analysis should be considered tentative. For both specimens we used casts available to us.
As noted, intact juvenile skulls of phylogenetic dwarfs are extremely rare. The two published skulls of juvenile P. falconeri (Ambrosetti 1968) and the skull of a juvenile P. tiliensis (depicted by Katsikosta and Theodorou 1994) were not available to us. To our knowledge, no juvenile skull of H. minor is known. Non-adult Malagasy hippos are restricted to subadult individuals.
Shape Analysis
Three-dimensional landmark data (Fig. 2) were acquired with a Microscribe multijoint 3D point digitizer. For practical reasons (specimen size) we digitized only the left side of each skull. Due to the extremely large size of mainland adult elephants, some specimens had to be digitized in different orientations. Datapoints were afterwards combined digitally to create a single specimen using the software DVRL.msi (Dorsal-Ventral-Right-Left fitting), a NYCEP Morphometrics Group software originally devised by David Reddy and revised by Ryan Raaum.
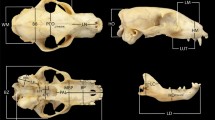
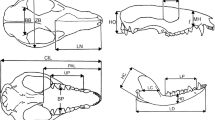
Landmarks used in this analysis. Landmarks on elephants are to the left, landmarks on the hippos are to the right. Landmarks on elephants: 1: tip of nasal process, 2: most anterior point of the nasal cavity at the incisive suture, 3: most lateral point of the nasal cavity along the external nares, 4: incisive-nasal suture along the external nares, 5: suture of the frontal, incisive and maxillary bones, 6: zygomatic process of the frontal bone, 7: dorsal process of the lacrimal bone, 8: median of nuchal line, 9: basisphenoid-presphenoid suture, 10: posterior palatine boarder, 11: medial boarder of condylar fossa, 12: lateral boarder of condylar fossa, 13: posterior point of the dental battery, 14: midpoint of the dental battery along the lingual line, 15: midpoint of the dental battery in buccal view, 16: anterior point of the dental battery, 17: alveolar border of the fan (premaxillaries including the tusk sheaths), 18: intermaxillary suture, 19: dorsal lip of infraorbital foramen, 20: suture between the zygomatic bone and the zygomatic process of the maxillary, 21: anterior projection of zygomatic process of temporal bone, 22: optic foramen, 23: external acoustic meatus, 24: parietal-temporal suture along the temporal line. 25: narrowest point of the skull along the temporal line. Landmarks on hippos: 1: median of nuchal crest, 2: parietal-temporal-occipital suture, 3: anterior projection of zygomatic process of temporal bone, 4: optic foramen, 5: tip of postorbital process, 6: lacrimal, 7: dorsal lip of infraorbital foramen, 8: posterior point of incisive at the incisive-maxilla suture, 9: widest point of foramen magnum, 10: jugular process, 11: mastoid process, 12: posterior projection of the zygomatic bone, 13: the most posterior element of the tooth row (M3 in adults), 14: the most anterior element of the premolar series, 15: buccal edge of the upper canine, 16: posterior point at midline of premaxillary-maxillary suture, 17: posterior edge on the midline of the palatine, 18: basion
Although our goal was to capture shape as best as possible, several potentially important landmarks had to be dropped because they were not preserved in all fossil specimens. In particular, P. antiquus SMNS 32888 lacks its occipital condyles (Adam 1986), which obliged us to exclude that area from our analysis of the elephant skull. In hippos, a limiting factor was that some vault sutures are not visible in aged examples of the Malagasy hippopotamuses, which obliged us to omit some landmarks that are generally used to characterize the vault in mammals. Furthermore, the available Cypriot hippo (H. minor) skull does not preserve its premaxillary bones. Landmarks on those parts therefore had to be omitted as well.
Generalized procrustes analysis (GPA) and principal component analysis (PCA) were performed in MorphoJ version 1.06 (Klingenberg 2011) and the visualization of shape variation along principal components was performed both in MorphoJ 1.06 and Morphololgika 2.5 (O’Higgins and Jones 1998). Procrustes analysis standardizes for variation in location, scale, and orientation; the resulting shape coordinates allow for the comparison of shape across individuals and species. Centroid size (the square root of the sum of squared Euclidean distances from each landmark to their centroid; see below) is used as a measure of overall skull size (Dryden and Mardia 2008). Shape differences were visualized using Morphologika 2.5 (O’Higgins and Jones 1998) by warping wireframes to the extremes of the sample variation along each principal component. Subsequently, we used these wireframes as the basis for drawing schematic skulls for illustration.
Body Size and Skull Size
Most museum specimens of large mammals lack associated data concerning body weight, hampering explorations of the relationship between body size and other traits. Weston and Lister (2009) demonstrated the validity of using cranial size as a proxy for body mass in hippos. We here extended their approach, to elephants as well as hippos, and compared these taxa to mammals in general. We estimated the volume of each skull by the volume of a polygon defined by 17 surface points.
The 3D coordinates of these points were measured with a Microscribe for three fossil and twelve contemporary elephants (E. maximus), three fossil and nine contemporary hippopotamuses (H. amphibius), and a representative sample of 30 contemporary species from ten orders of placental mammals (see Online Resource 1). The skull volume of mainland P. antiquus (Pian’ dell’Olmo, Italy) was taken directly from Accordi and Palombo (1971). Triangulated meshes were constructed with Rhino3D software.
In order to account for the phylogenetic structure in our sample of 30 contemporary mammalian species (for species list, see Online Resource 1), we used phylogenetic generalized least squares (PGLS; R package evomap, Smaers 2014) and a time-calibrated molecular phylogenetic tree (Bininda-Emonds et al. 2007; Revell 2009) to estimate allometric relationships.
Body mass estimates for fossil species based on postcranial elements were taken from Weston and Lister (2009) and Lomolino et al. (2013). Mean body mass data of living species were taken from Smith et al. (2003). Body mass of E. maximus and H. amphibius in the ontogenetic regressions were reconstructed following Weston and Lister (2009) and the data of Martin (2005) for H. amphibius and those of Kurt and Kumarasinghe (1998) for E. maximus.
Results
Relation Between Body Size and Skull Size
Skull size and body size within adult terrestrial placental mammals scaled almost isometrically, with a PGLS major axis slope of 0.906 for the log-transformed variables (Fig. 3). Similarly, in the ontogenetic samples of H. amphibius and E. maximus skull size and body mass had a major axis slope of 0.9221 and 1.2262, respectively. An approximately isometric relationship between skull and body size seems to be characteristic of placental mammals, with a tendency towards negative allometry in hippos and towards positive allometry in elephants. Full-grown elephants have a larger skull than the average mammalian trend suggests, whereas hippos have smaller skulls than predicted for their body size.
Regressions of skull size (volume in mm3; log10 transformed) onto body mass (in g; log10 transformed). Black line: regression for 30 mammalian species (see Online Resource 1), corrected for phylogeny using PGLS. Red line: ontogenetic regression for living Asian elephants (E. maximus). Green line: ontogenetic regression for living African hippos (H. amphibius). Included as separate dots outside the regression are the fossil elephants (orange squares) and hippos (green squares) used in this study (Table 1). (Color figure online)
Skull Shape in Dwarf Elephants
Nearly 39% of total shape variation is explained by the first principal component (PC1). This component is affected by the size of the fan (i.e., the greatly expanded premaxillaries including tusk sheaths), the development of the forehead and the position of the skull’s centre of gravity (Fig. 4a). In large adults, low PC1 values are associated with large tusk sheaths, relatively short foreheads, and posteriorly-shifted centre of gravity. High PC1 values are associated with small tusk sheaths and relatively long foreheads as is typically seen in calves and juveniles. The second principal component (PC2), accounting for 11% of total variation, is related to the shape of the skull’s lateral profile. At high PC2 values, the lateral profile of the face is rounded (slightly convex), whereas at low PC2 values the profile is compressed parallel to the basicranial plane. Mainland Palaeoloxodon is well separated from Elephas and Loxodonta not only by its extremely flattened lateral profile but also by its huge, elongated fan and short forehead. Insular representatives of Palaeoloxodon, on the contrary, have more rounded faces, approaching juvenile Elephas and Loxodonta in this respect (Fig. 4a). Palaeoloxodon, the largest elephant of our dataset, appears to mimic very young elephants by having, again, a flattened lateral profile. This is also the case for the largest Loxodonta, located at the right lower end of the adult cluster in Fig. 4a.
Morphometric analysis of cranial shape in elephants. a Scatter plot of the first two PCs of cranial shape. b Scatter plot of the first versus the third PC. c Regression of the first five shape PCs on centroid size. The individual scores for these allometric shape features are plotted against centroid size. d Regression of the first 5 PCs on approximated age expressed as dental stages. The scores for these age-related shape features are plotted against centroid size
The scatter plot of PC1 versus PC3 (8% of total variance) separates Elephas from Loxodonta (Fig. 4b). PC3 mainly reflects the length of the face and the position of the widest part of the skull. In Loxodonta the widest part is across the orbits, while in Elephas it is at the parietal-occipital region. PC3 also reflects the relative width of the fan. In Loxodonta the fan is short and broad, in Elephas long and narrow. Mainland Palaeoloxodon is more like Elephas in this respect with its relatively narrow face. The only outlier amongst Loxodonta (AMNH (M) 113819) is the largest extant elephant in our dataset, perhaps suggesting an allometric effect. The dwarf elephants separate from Elephas and mainland Palaeoloxodon, with PC3 scores comparable to those of Loxodonta, but have shorter foreheads and longer fans, comparable to those of Palaeoloxodon.
Figure 4c shows the regression scores of the first five PCs (69% of the total variance) on centroid size (orthogonal projection of the individuals on the vector of regression coefficients, reflecting the individual expression of allometric shape features). The ontogenetic trajectory of size-shape change extends linearly into the static allometric variation of adult elephants (note that this contrasts with the pattern in the first two PCs). All elephants follow the same trend of increased allometric shape scores with increasing size. Importantly, Palaeoloxodon species, mainland as well as insular, remain distinct from both Elephas and Loxodonta and follow their own trajectory, which lies at all sizes above that of the extant elephants. The shape of the least size-reduced dwarf species, P. ‘mnaidriensis’, is intermediate between that of P. recki and subadult P. antiquus. The female dwarf P. falconeri is similar in shape to juvenile elephants but at a smaller size.
The regression of the first five principal components on age (approximated by dental developmental stage; specimens with unassigned dental stage excluded) shows that none of the dwarf species aligns with extant juveniles due to their considerably higher shape scores, except for the female P. falconeri (Fig. 4d). The least size-reduced dwarf species, P. ‘mnaidriensis’, has a similar shape as mainland P. recki.
Skull Shape in Dwarf Hippos
PC1 accounts for 39% of total variation and is mainly related to the elongation of the muzzle (Fig. 5a). Low PC1 scores correspond to a relatively short muzzle while high PC1 scores correspond to a relatively elongated muzzle. The muzzles of both H. amphibius and C. liberiensis become more elongated during ontogeny, but neonates of H. amphibius start with a relatively more elongated muzzle and, eventually, end up with a proportionally much longer muzzle. PC2, accounting for 11% of total variation, is mainly related to the width and height of the rostral part of the muzzle. Low PC2 scores are associated with a large distance between the canines and elevated maxillary bones. In both H. amphibius and C. liberiensis, the muzzle becomes wider and higher as they age. H. madagascariensis, by contrast, has a much shorter muzzle, comparable to subadult H. amphibius, but at the same time narrower and flatter, distinguishing it from the latter. The other Malagasy dwarf hippo, H. lemerlei, has a muzzle as long as that of adult H. amphibius, but at the same time very narrow and low, unlike any other hippo. The muzzle of H. minor is even shorter and clusters with very young Hippopotamus individuals.
Morphometric analysis of cranial shape in hippos. a Scatter plot of the first two PCs of cranial shape. b Regression of the first 5 PCs of cranial shape on centroid size. The scores for these allometric shape features are plotted against centroid size. c Regression of the first five shape PCs on approximated age expressed as Hippopotamus amphibius years. The scores for these age-related shape features are plotted against centroid size
All three insular hippos have relatively narrow and low muzzles, more closely resembling juvenile than adult Hippopotamus in this respect. As shown by the regression analysis of the first five principal components (73% of total variance; Fig. 5b) on centroid size, modern hippos extend their ontogenetic trajectory into the pattern of adult static allometry, analogously to elephants (Fig. 4c). The two Malagasy dwarf species are similar in overall shape to the largest juvenile H. amphibius and the smallest adult H. amphibius but at a smaller size. H. minor, by contrast, resembles the largest juvenile C. liberiensis and very young H. amphibius at a marginally smaller size.
The regression analysis of the first five principal components on dental developmental stage (Fig. 5c; specimens with unassigned dental stage excluded) shows that the adult Malagasy hippos cluster within (in the case of H. lemerlei) or slightly below (in the case of H. madagascariensis) adult H. amphibius. Their shape score differs from that of juveniles, except for the largest. H. minor, and aligns with adult C. liberiensis.
Discussion
Even though skull size to body size is almost isometric within elephants and hippos, they differ in the allometric relationships between these variables. Elephants—extant species as well as insular dwarf species—have relatively large skulls, resulting from the pneumatic bone tissue that provides muscle attachment areas to support the large tusks. For example the Sicilian dwarf species P. falconeri, with an adult weight comparable to that of a newborn modern elephant, has a surprisingly large skull. The opposite trend is observed in dwarf hippos, all of which have relative skull sizes comparable to those of their mainland relatives.
The differences in shape between Loxodonta and Elephas as described by PC2 are in line with Todd’s (2010) observations. In frontal view (Todd 2010, Fig. 1), the frontals (Todd’s feature C) of Loxodonta are broad, while they are narrow in Elephas. The premaxillary fan is very large and flares widely at its inferior end in Loxodonta, but is smaller and parallel-sided for most of its length in Elephas. The widest part of the skull (Todd’s feature I) of Loxodonta is at the level of the orbits, whereas it is in the parietal-occipital region in Elephas. The dwarf elephants cluster in this respect more closely with Loxodonta. This might, at least in part, explain why Aguirre (1969) placed P. mnaidriensis in the genus Loxodonta, based on a nearly complete skull (MPG 1). Finally, part of the observed generic differences seem to have an allometric component, as the skull shape of extremely large Loxodonta and Elephas approaches that of Palaeoloxodon in this respect, whereas dwarfed Palaeoloxodon approaches Loxodonta.
Interestingly, the ontogenetic development of skull shape in elephants continues into the allometric trajectory of adults (Fig. 4c), except for the relative size of the forehead (PC1) and the degree of flatness of the face in lateral profile (PC2) (Fig. 4a). For example, large adult specimens have relatively large tusk sheaths, short foreheads, and a posteriorly positioned centre of gravity of the skull. This might also be related to the unique anatomy of their dental battery. Dwarf elephants not only have smaller teeth, but also teeth composed of fewer lamellae (e.g. Lister 1996). Based on the above observations, skull size seems free to vary within elephants without involving any major reorganization, whether this results from an earlier cessation of growth or a slower growth rate. The relative flattening in large-sized elephants is related to the addition of pneumatic bone mass in the ventral part of the skull as well. This is apparently due to an allometric effect, as is the width of the face and the position of the widest part of the skull, these being the main features that separate Loxodonta from Elephas plus the largest Loxodonta.
Our observations may at least partly explain why elephants were so successful as island colonizers. Within Late Neogene/Quaternary time, several genera of elephantids, including Elephas, Mammuthus, Palaeoloxodon, Stegodon, Stegolophodon, and Stegoloxodon, managed to establish viable populations on islands, which subsequently evolved into endemic dwarf species. They also exhibited some of the most spectacular cases of dwarfing among large-bodied mammals: in addition to P. falconeri of Sicily and P. cypriotes of Cyprus, already discussed above, Mammuthus creticus (Bate, 1907) of Crete achieved a mass only 4% of that of its ancestor, M. meridionalis (Nesti, 1825), while Stegodon sumbaensis Sartono 1979 of Sumba (Lesser Sunda Islands, Indonesia) collapsed its body size to just 8% of that of S. ganesa (Van der Geer et al. 2016). Apparently, these massive size reductions did not require major structural changes in the skull. The intermediate position of the medium-sized P. ‘mnaidriensis’ relative to the small P. falconeri in PC1 and PC3 seems to indicate that skull shape does not change dramatically during the initial degrees of dwarfing, whereas it does so during extreme dwarfing, in accordance with Lister’s (1996) observation that initially, dwarfism would be merely an allometric stunting process but that in the longer term, natural selection would produce further changes and adaptations under evolutionary pressures exerted by the local environment.
Regarding hippos, our results confirm that the mainland dwarf hippo Choeropsis is not an ontogenetically scaled down version of Hippopotamus. This was demonstrated earlier by Weston (2003), who analyzed the allometric growth of extant hippos using traditional morphometrics and concluded that the morphological differences between the two extant hippo species are not merely the result of ontogenetic scaling and that C. liberiensis is not an example of a dwarfed species. The feature displaying the largest amount of morphological variation in hippos is muzzle shape. This is not surprising, as facial elongation in mammals is directly related to size (Cardini and Polly 2013), and in most taxa the first principal component (PC1) expresses muzzle length. There is a constraint on facial length relative to adult size, so that small mammals are in general more brachycephalic than large mammals (Cardini and Polly 2013). Our analysis confirms that juveniles of both extant hippo species have shorter muzzles than adults, and that elongation of the muzzle increases during their ontogenetic development. Even as an adult, though, C. liberiensis has a shorter muzzle than H. amphibius. Furthermore, its muzzle is situated higher on the skull than in the latter species.
Each of the three insular dwarf hippos in our dataset exhibits a different degree of muzzle shortening. H. minor has a very short muzzle, H. madagascariensis has a moderately short muzzle and H. lemerlei exhibits practically no shortening at all but an anterior narrowing instead. The very short muzzle of H. minor is a feature that resembles the situation seen in both juvenile Hippopotamus and adult Choeropsis. As indicated above, the short muzzle of H. minor could simply be a function of its absolutely small size. Interestingly, the extreme reduction of muzzle length in adult H. minor is associated with the loss of the fourth upper premolar in many specimens. Therefore, it appears that—in order to accommodate its dental battery in a shorter muzzle—the Cypriot hippo had to lose a tooth locus. This stands in sharp contrast to insular elephants, which shortened the length of individual cheek teeth by reducing the number of lamellae.
The muzzle of H. lemerlei is peculiarly elongated for a hippo of its size, and is anteriorly narrow and low, with the nostrils positioned below the level of the orbits. According to Stuenes (1989), the two species of Malagasy hippo were adapted to different ecological regimes (semiaquatic H. lemerlei versus terrestrial H. madagascariensis), as reflected in contrasting styles of tooth wear. Differences in muzzle configuration might reflect this evolutionary divergence.
As a general observation, the evolution of insular dwarfism seems to echo phylogeny (that is, island dwarfs may resemble earlier members of the lineage), but the signal can be misleading—the fallacy of recapitulation. Therefore, caution is appropriate when using morphometric data to extrapolate aspects of the phenotype of a potential founder species. Thus, the skull of H. minor does not resemble that of its mainland relative, H. amphibius, but rather that of unrelated C. liberiensis, which in turn is morphologically close to Pliocene and Early Pleistocene Hexaprotodon (Coryndon 1977). Similarly, both Malagasy hippos deviated in skull shape from their presumed ancestor, H. amphibius, and convergently display features—especially in H. madagascariensis—that are otherwise typical for the genus Hexaprotodon (Stuenes 1989). A similar trend can be observed in the Sardinian dwarf canid Cynotherium sardous. Although this latter taxon is an offshoot of Xenocyon (a derived hypercarnivorous Canis-like species), it was earlier claimed to be related to more primitive forms such as Eucyon and early Canis (see discussion in Lyras et al. 2006).
An especially interesting case concerns the phylogenetic position of the dwarf hominin of Flores, Homo floresiensis. Although this insular hominin has been known for more than a decade, its phylogenetic status remains contentious (for a recent analysis see Argue et al. 2017). Morphometric analyses of cranial material have aligned it either with Homo erectus (e.g. Argue et al. 2006; Lyras et al. 2009; Baab and McNulty 2009; Kaifu et al. 2011; Zeitoun et al. 2016) or an earlier form close to Homo habilis (e.g. Argue et al. 2009; Martinez and Hamsici 2008). However, assuming that hominins dwarf along trajectories similar to those of other dwarf mammals, the apparent similarities might be simply due to general patterns of shape change during dwarfing and have nothing to do with actual ancestral features (Weston and Lister 2009). In other words, the morphology of the Flores dwarf hominin may not indicate any particularly close relationship with any extinct hominin, but such resemblances may simply be the result of convergence. For this reason, features that are independent of skull shape are crucial for diagnostic purposes. Examples include external brain morphology and dental characters, which place the “hobbit” in the genus Homo (e.g. Falk et al. 2007 and van den Bergh et al. 2016 respectively).
Conclusions
Three-dimensional morphometric quantification of cranial shape in selected insular dwarf species of proboscideans (Palaeoloxodon ‘mnaidriensis’, P. falconeri) and hippos (Hippopotamus madagascariensis, H. lemerlei, H. minor) revealed that their skulls do not scale isometrically and are thus not simply scaled-down versions of their mainland source species. Instead, in the hippos the muzzle becomes anteriorly low and, in the Cypriot pygmy hippo in particular, notably foreshortened—much more so than would be expected for its skull size. This requires a major restructuring of the dental battery, including the loss of a premolar. In dwarf elephants the tusk sheaths become proportionally smaller, the forehead broader and the centre of gravity of the skull moves forward during phyletic dwarfing. This does not require a major restructuring of skull elements but is a function of size. Our morphometric analysis further indicates that adult skulls of these insular dwarf taxa differed substantially from those of mainland juveniles, including those of the mainland dwarf taxa (Choeropsis liberiensis and Loxodonta cyclotis respectively). The pedomorphic aspect of some features, such as the shorter muzzle in H. minor, is best explained as a correlate of its smaller skull size.
References
Accordi, F. S., & Palombo, M. R. (1971). Morfologia endocranica degli elephanti nani pleistocenici de Spinagalo (Siracusa) e comparazione con l´endocranio de Elephas antiquus. Rediconti dell´Accademia Nazionale del Lincei (Series 8), 51, 111–124.
Adam, K. D. (1986). Fossilfunde aus den Cannstatter Sauerwasserkalken. In K. D. Adam, W. Reif & E. Wagner (Eds.), Seugnisse des Urmenschen aus den Cannstatter Sauerwasserkalken (Vol. 11, pp. 25–61). Baden-Württemberg: Fundber.
Aguirre, E. (1969). Revision sistematica de los Elephantidae, por su morfologia y morfometria dentaria. Estudios Geologicos, 25, 317–367.
Ambrosetti, P. (1968). The Pleistocene dwarf elephant of Spinagallo. Geologica Romana, 7, 277–398.
Argue, D., Donlon, D., Groves, C., & Wright, R. (2006). Homo floresiensis: Microcephalic, pygmoid, Australopithecus or Homo? Journal of Human Evolution, 51, 360–374.
Argue, D., Groves, C. P., Lee, M. S. Y., & Jungers, W. L. (2017). The afffinities of Homo floresiensis based on phylogenetic analyses of cranial, dental, and postcranial characters. Journal of Human Evolution, 197, 107–133.
Argue, D., Morwood, M., Sutikna, T., Jatmiko, W., & Saptomo, E. (2009). Homo floresiensis: A cladistic analysis. Journal of Human Evolution, 57, 623–639.
Athanassiou, A., Herridge, V., Reese, D. S., et al. (2015). Cranial evidence for the presence of a second endemic elephant species on Cyprus. Quaternary International, 379, 47–57.
Baab, K. L., & McNulty, K. P. (2009). Size, shape, and asymmetry in fossil hominins: The status of the LB1 cranium based on 3D morphometric analyses. Journal of Human Evolution, 57, 608–622.
Bininda-Emonds, O. R. P., Cardillo, M., Jones, K. E., MacPhee, R. D. E., Beck, R. M. D., Grenyer, R., Price, S. A., Vos, R. A., Gittleman, J. L., & Purvis, A. (2007). The delayed rise of present-day mammals. Nature, 446, 507–512.
Boekschoten, G. J., & Sondaar, P. Y. (1972). On the fossil mammalia of Cyprus, I and II. Proceedings van de Koninklijke Nederlandse Akademie van Wetenschappen, 75, 306–338.
Boisserie, J. R. (2005). The phylogeny and taxonomy of Hippopotamidae (Mammalia: Artiodactyla): A review based on morphology and cladistic analysis. Zoological Journal of the Linnean Society, 143, 1–26.
Cardini, A., & Polly, P. D. (2013). Larger mammals have longer faces because of size-related constraints on skull form. Nature Communications, 4, 2458. https://doi.org/10.1038/ncomms3458.
Chiozzi, G., Bardelli, G., Ricci, M., De Marchi, G., & Cardini, A. (2014). Just another island dwarf? Phenotypic distinctiveness in the poorly known Soemmerring’s Gazelle, Nanger soemmerringii (Cetartiodactyla: Bovidae), of Dahlak Kebir Island. Biological Journal of the Linnean Society, 111, 603–620.
Coryndon, S. C. (1977). The taxonomy and nomenclature of the Hippopotamidae (Mammalia, Artiodactyla) and a description of two new fossil species. The nomenclature of the Hippopotamidae. Proceedings of the Koninklijke Nederlandse Akademie van Wetenschappen B, 80, 61–88.
Dryden, I. L., & Mardia, K. M. (2008). Statistical shape analysis. Chicester: Wiley.
Evans, A. R., Jones, D., Boyer, A. G., et al. (2012). The maximum rate of mammal evolution. Proceedings of the National Academy of Sciences of the United States of America, 109, 4187–4190.
Falk, D., Hildebolt, C., Smith, K., et al. (2007). Brain shape in human microcephalics and Homo floresiensis. Proceedings of the National Academy of Sciences of the USA, 104, 2513–2518.
Ferretti, M. P. (2008). The dwarf elephant Palaeoloxodon mnaidriensis from Puntali Cave, Carini (Sicily; late Middle Pleistocene): Anatomy, systematics and phylogenetic relationships. Quaternary International, 182, 90–108.
Fovet, W., Faure, M., & Guerin, C. (2011). Hippopotamus guldbergi n. sp.: révision du statut d'Hippopotamus madagascariensis Guldberg, 1883, après plus d'un siècle de malentendus et deconfusions taxonomiques. Zoosystema, 33, 61–82.
Goodman, S. M., & Jungers, W. L. (2014). Extinct Madagascar: Picturing the Island’s Past. Chicago: University of Chicago Press.
Herridge, V. L. (2010). Dwarf elephants on Mediterranean islands: A natural experiment in parallel evolution. PhD Thesis, University College, London.
Houtekamer, J. L., & Sondaar, P. Y. (1979). Osteology of the fore limb of the Pleistocene dwarf hippopotamus from Cyprus with special reference to phylogeny and function. Proceedings van de Koninklijke Nederlandse Akademie van Wetenschappen, 82, 411–448.
Iinuma, Y. M., Tanaka, S., Kawasaki, K., Kuwajima, T., Nomura, H., Suzuki, M., & Ohtaishi, N. (2004). Dental incremental lines in Sika Deer (Cervus nippon); polarized light and fluorescence microscopy of ground sections. Journal of Veterinary Medical Science, 66, 665–669.
Kaifu, Y., Baba, H., Sutika, T., Morwood, M. J., Kubo, D., Saptomo, W., Jatmiko, E., Due Awe, R., & Djubiantono, T. (2011). Craniofacial morphology of Homo floresiensis: Description, taxonomic affinities, and evolutionary implication. Journal of Human Evolution, 61, 644–682.
Katsikosta, N., & Theodorou, G. (1994). Conservation and reconstruction of a juvenile skull of Palaeoloxodon antiquus falconeri from Charkadio Cave, Tilos island (Dodecanese, Greece). Bulletin of the Speleological Society of Greece, 21, 263–628.
Klingenberg, C. P. (2011). MorphoJ: An integrated software package for geometric morphometrics. Molecular Ecology Resources, 11, 353–357.
Kolb, C., Scheyer, T. M., Veitschegger, K., et al. (2015). Mammalian bone palaeohistology: A survey and new data with emphasis on island forms. PeerJ, 3, e1358. https://doi.org/10.7717/peerj.1358.
Kubo, M. O., Fujita, M., Matsu’ura, S., Kondo, M., & Suwa, G. (2011). Mortality profiles of late Pleistocene deer remains of Okinawa Island: Evidence from the Hananda-Gama cave and Yamashita-cho cave I sites. Anthropological Series, 119, 183–201.
Kurt, F., & Kumarasinghe, J. C. (1998). Remarks on body growth and phenotypes in Asian elephant Elephas maximus. Acta Theriologica, 43, 135–153.
Laws, R. M. (1966). Age criteria for the African elephant Loxodonta africana. East African Wildlife Journal, 4, 1–37.
Laws, R. M. (1968). Dentition and ageing of the Hippopotamus. East African Wildlife Journal, 6, 19–52.
Lister, A. M. (1989). Rapid dwarfing of red deer on Jersey in the Last Interglacial. Nature, 342, 539–542.
Lister, A. M. (1996). Dwarfing in island elephants and deer: Processes in relation to time of isolation. Symposia of the Zoological Society of London, 69, 277–292.
Lomolino, M. V., Sax, D. F., Palombo, M. R., & van der Geer, A. A. E. (2012). Of Mice and mammoths: Evaluations of causal explanations for body size evolution in insular mammals. Journal of Biogeography, 39, 842–854.
Lomolino, M. V., van der Geer, A. A. E., Lyras, G. A., Palombo, M. R., Sax, D. F., & Rozzi, R. (2013). Of mice and mammoths: Generality and antiquity of the island rule. Journal of Biogeography, 40, 1427–1439.
Lyras, G. A., Dermitzakis, M. D., van der Geer, A. A. E., & de Vos, J. (2009). The origin of Homo floresiensis and its relation to evolutionary processes under isolation. Anthropological Science, 117, 33–43.
Lyras, G. A., van der Geer, A. A. E., & Rook, L. (2010). Body size of insular carnivores: Evidence from the fossil record. Journal of Biogeography, 37, 1007–1021.
Lyras, G. A., van der Geer, A. E., Dermitzakis, M., & de Vos, J. (2006). Cynotherium sardous, an insular canid (Mammalia: Carnivora) from the Pleistocene of Sardinia (Italy), and its origin. Journal of Vertebrate Paleontology, 26, 735–745.
Martin, R. B. (2005). The Transboundary Mammal Project of the Ministry of Environment and Tourism, Namibia (p. 74). Windhoek: The Namibia Nature Foundation.
Martinez, A., & Hamsici, O. (2008). Who is LB1? Discriminant analysis for the classification of specimens. Pattern Recognition, 41, 3436–3441.
Masters, J. C., Génin, F., Silvestro, D., Lister, A. M., & DelPero, M. (2014). The red island and the seven dwarfs: Body size reduction in Cheirogaleidae. Journal of Biogeography, 41, 1833–1847.
Millien, V. (2006). Morphological evolution is accelerated among island mammals. PLoS Biology, 4, e321.
Mitteroecker, P., Gunz, P., & Bookstein, F. L. (2005). Heterochrony and geometric morphometrics: A comparison of cranial growth in Pan paniscus versus Pan troglodytes. Evolution & Development, 7, 244–258.
Mitteroecker, P., Gunz, P., Windhager, S., & Schaefer, K. (2013). A brief review of shape, form, and allometry in geometric morphometrics, with applications to human facial morphology. Hystrix, The Italian Journal of Mammalogy, 24, 59–66.
O’Higgins, P., & Jones, N. (1998). Morphologika a program for the analysis of 3-dimensional shape variation using landmarks. http://discovery.ucl.ac.uk/id/eprint/172805.
Palkopoulou, E., Lipson, M., Mallick, S., Nielsen, S., Rohland, N., Baleka, S., et al. (2018). A comprehensive genomic history of extinct and living elephants. Proceedings of the National Academy of Sciences of the USA. https://doi.org/10.1073/pnas.1720554115.
Palombo, M. R. (2001). Paedomorphic features and allometric growth in the skull of Elephas falconeri from Spinagallo (Middle Pleistocene, Sicily). In G. Cavaretta, P. Gioia, M. Mussi & M. R. Palombo (Eds), The World of Elephants. Proceedings of the First International Congress, Rome, (Vol. 16–20, pp. 492–496). Roma: CNR.
Penin, X., Berge, C., & Baylac, M. (2002). Ontogenetic study of the skull in modern humans and the common chimpanzees: Neotenic hypothesis reconsidered with a tridimensional procrustes analysis. American Journal of Physical Anthropology, 118, 50–62.
Pergams, O. R. W., & Ashley, M. V. (1999). Rapid morphological change in island deer mice. Evolution, 53, 1573–1581.
Revell, L. J. (2009). Size-correction and principal components for interspecific comparative studies. Evolution, 63, 3258–3268.
Roca, A. L., Georgiadis, N., Pecon-Slattery, J., & O’Brien, S. J. (2001). Genetic evidence for two species of elephant in Africa. Science, 293, 1473–1477.
Roth, V. L. (1984). How elephants grow: Heterochrony and the calibration of developmental stages in some living and fossil species. Journal of Vertebrate Paleontology, 4, 126–145.
Roth, V. L. (1992). Inferences from allometry and fossils: Dwarfing of elephants on islands. Oxford Surveys in Evolutionary Biology, 8, 259–288.
Roth, V. L. (1993). Dwarfism and variability in the Santa Rosa Island mammoth (Mammuthus exilis): An interspecific comparison of limb-bone sizes and shapes in elephants. In F. G. Hochberg (Ed.), Third California Islands Symposium (pp. 433–442). Santa Barbara: Santa Barbara Museum of Natural History.
Roth, V. L., & Shoshani, J. (1988). Dental identification and age determination in Elephas maximus. Journal of Zoology, 214, 288–567.
Sikes, K. S. (1967). The African elephant, Loxodonta africana: A field method for the estimation of age. Journal of Zoology, 154, 235–248.
Smaers, J. B. (2014). Evomap: R-package for the evolutionary mapping of continuous traits. Available at Github: https://github.com/JeroenSmaers/evomap.
Smith, F. A., Lyons, S. K., Ernest, S. K. M., Jones, K. E., Kaufman, D. M., Dayan, T., Marquet, P. A., Brown, J. H., & HaskelI, J. P. (2003). Body mass of late Quaternary mammals. Ecology, 84, 3402.
Sondaar, P. Y. (1977). Insularity and its effect on mammal evolution. In M. K. Hecht, P. C. Goody & B. M. Hecht (Eds), Major Patterns in Vertebrate Evolution (pp. 671–707). New York: Plenum Publications Corporation.
Sondaar, P. Y. (1994). Paleoecology and evolutionary patterns in horses and island mammals. Historical Biology, 8, 1–13.
Stuenes, S. (1989). Taxonomy, habits, and relationships of the subfossil Madagascan Hippopotami Hippopotamus lemerlei and H. madagascariensis.. Journal of Vertebrate Paleontology, 9, 241–268.
Todd, N. (2010). Qualitative Comparison of the Cranio-Dental Osteology of the Extant Elephants, Elephas Maximus (Asian Elephant) and Loxodonta africana (African Elephant). The Anatomical Record, 293, 62–73.
van den Bergh, G. D., Kaifu, Y., Kurniawan, I., et al. (2016). Homo floresiensis-like fossils from the early Middle Pleistocene of Flores. Nature, 534, 245–248.
van den Bergh, G. D., Meijer, H. J. M., Awe, R. D. et al. (2009). The Liang Bua faunal remains: A 95k.yr. sequence from Flores, East Indonesia. Journal of Human Evolution, 57, 527–537.
van der Geer, A., Lyras, G., de Vos, J., & Dermitzakis, M. (2010). Evolution of Island Mammals: Adaptation and Extinction of Placental Mammals on Islands (p. 479). Oxford: Wiley-Blackwell Publishing.
van der Geer, A. A. E. (2005). Island ruminants and the evolution of parallel functional structures. In E. Crégut (Ed.) Les ongules holarctiques du Pliocene et du Pleistocene. Actes Colloque International Avignon, (Vol. 19–22, pp. 231–240) Quaternair (hors-serie 2).
van der Geer, A. A. E. (2014). Parallel patterns and trends in functional structures in island mammals. Integrative Zoology, 9, 167–182.
van der Geer, A. A. E., Lyras, G. A., Lomolino, M. V., Palombo, & Sax, M. R., D.F (2013). Body size evolution of palaeo-insular mammals: Temporal variations and interspecific interactions. Journal of Biogeography, 40, 1440–1450.
van der Geer, A. A. E., Lyras, G. A., van den Hoek Ostende, L. W., de Vos, J., & Drinia, H. (2014). A dwarf elephant and a rock mouse on Naxos (Cyclades, Greece) with a revision of the palaeozoogeography of the Cycladic Islands (Greece) during the Pleistocene. Palaeogeography, Palaeoclimatology, Palaeoecology, 404, 133–144.
van der Geer, A. A. E., van den Bergh, G. D., Lyras, G. A., Prasetyo, U. W., Awe Due, R., Setiyabudi, E., & Drinia, H. (2016). The effect of area and isolation on insular dwarf proboscideans. Journal of Biogeography, 43, 1656–1666.
van Heteren, A. H. (2008). Homo floresiensis is an island form. PalArch’s Journal of Vertebrate Palaeonotology, 5(2), 1–12.
Van Valen, L. (1973). Body size and numbers of plants and animals. Evolution, 27, 27–35.
Weston, E. M. (1998). A biometrical analysis of evolutionary change within the Hippopotamidae. PhD thesis, University of Cambridge, England.
Weston, E. M. (2003). Evolution of ontogeny in the hippopotamus skull: Using allometry to dissect developmental change. Biological Journal of the Linnean Society, 80, 625–638.
Weston, E. M., & Lister, A. M. (2009). Insular dwarfism in hippos and a model for brain size reduction Homo floresiensis. Nature, 459, 85–88.
Zeitoun, V., Barriel, V., & Widianto, H. (2016). Phylogenetic analysis of the calvaria of Homo floresiensis. Comptes Rendus Palevol, 15, 555–568.
Acknowledgements
We thank Steven van der Mije and Wendy van Bohemen (RMNH), Eileen Westwig and Neil Duncan (AMNH), Laurence Heaney and the late William Stanley (FMNH), Carolina di Patti (MGG), Oliver Hampe, Frieder Mayer and Nora Lange (MFN), Christine Argot and Christine Lefèvre (MNHN), Rainer Brocke and Christine Hertler (SFN), Reinhard Ziegler (SMNS), Darrin Lunde and Nicole Edmison (USNM), Chloe Adamopoulou (ZMUA), Pasquale Raia and Mariella Del Re (MPUN) and Pip Brewer (Natural History Museum, London) for allowing us to study the skulls in their care and their assistance when skulls were too heavy to handle two-handed. We further thank Rutger Vos for clarifying PGLS regressions, and Gert van den Bergh (Centre for Archaeological Science, University of Wollongong), Maria Rita Palombo (‘La Sapienza’ University of Rome), Athanassios Athanassiou (Hellenic Ministery of Culture), Adrian Lister, Chris Stringer and Victoria Herridge (Natural History Museum, London) for discussions we had on island dwarfs.
Funding
GL received support from the SYNTHESYS Project (GB-TAF-6355 and FR-TAF-6549). The research of AVDG has been co-financed by the European Union (European Social Fund—ESF) and Greek national funds through the Operational Program “Education and Lifelong Learning” of the National Strategic Reference Framework (NSRF)—Research Funding Program: THALIS—UOA-Island biodiversity and cultural evolution: examples from the Eastern Mediterranean, Madagascar, Mauritius and Philippines during the past 800,000 years (MIS375910, KA:70/3/11669).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
van der Geer, A.A.E., Lyras, G.A., Mitteroecker, P. et al. From Jumbo to Dumbo: Cranial Shape Changes in Elephants and Hippos During Phyletic Dwarfing. Evol Biol 45, 303–317 (2018). https://doi.org/10.1007/s11692-018-9451-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11692-018-9451-1