Abstract
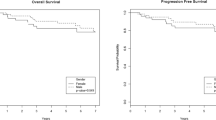
Dasatinib is a highly effective second-generation tyrosine kinase inhibitor used to treat chronic myeloid leukemia (CML). In 2007, a pivotal phase-2 study of dasatinib as second-line treatment was initiated in 140 Chinese CML patients. This report from the 4-year follow-up revealed that 73% of 59 patients in chronic phase (CML-CP) and 32% of 25 patients in accelerated phase (CML-AP) remained under treatment. The initial dosage of dasatinib for CML-CP and CML-AP patients were 100 mg once daily and 70 mg twice daily (total = 140 mg/ day), respectively. The cumulative major cytogenetic response (MCyR) rate among patients with CML-CP was 66.1% (versus 50.8% at 18 months), and the median time to MCyR was 12.7 weeks. All CML-CP patients who achieved MCyR after a 4-year follow-up also achieved a complete cytogenetic response. The cumulative complete hematological response (CHR) rate among patients with CML-AP was 64% (16/25), with three CML-AP patients achieving CHR between 18 months and 4 years of follow-up; the median time to CHR was 16.4 weeks. The adverse event (AE) profile of dasatinib at 4 years was similar to that at 6 and 18 months. The most frequently reported AEs (any grade) included pleural effusion, headache, and myelosuppression. These long-term follow-up data continue to support dasatinib as a second-line treatment for Chinese patients with CML.
Similar content being viewed by others
References
de Lavallade H, Apperley JF, Khorashad JS, Milojkovic D, Reid AG, Bua M, Szydlo R, Olavarria E, Kaeda J, Goldman JM, Marin D. Imatinib for newly diagnosed patients with chronic myeloid leukemia: incidence of sustained responses in an intention-to-treat analysis. J Clin Oncol 2008; 26(20): 3358–3363
NCCN Clinical Practice Guidelines in Oncology(NCCN Guidelines ®). Chronic Myeloid Leukemia. Version 1. 2018
Bristol-Myers Squibb company. Sprycel (dasatinib) prescribing information. Princeton, NJ: Bristol-Myers Squibb company, 2017
Huang XJ, Hu JD, Li JY, Jin J, Meng FY, Shen ZX, Liu T, Wu DP, Wang JM, Wang JX. Study on efficiency and safety of dasatinib in Chinese patients with chronic myelogenous leukemia who are resistant or intolerant to imatinib. Chin J Hematol (Zhonghua Xue Ye Xue Za Zhi) 2012; 33(11): 889–895 (in Chinese)
Jabbour E, Kantarjian HM, Saglio G, Steegmann JL, Shah NP, Boqué C, Chuah C, Pavlovsky C, Mayer J, Cortes J, Baccarani M, Kim DW, Bradley-Garelik MB, Mohamed H, Wildgust M, Hochhaus A. Early response with dasatinib or imatinib in chronic myeloid leukemia: 3-year follow-up from a randomized phase 3 trial (DASISION). Blood 2014; 123(4): 494–500
Shah NP, Guilhot F, Cortes JE, Schiffer CA, le Coutre P, Brümmendorf TH, Kantarjian HM, Hochhaus A, Rousselot P, Mohamed H, Healey D, Cunningham M, Saglio G. Long-term outcome with dasatinib after imatinib failure in chronic-phase chronic myeloid leukemia: follow-up of a phase 3 study. Blood 2014; 123(15): 2317–2324
Shah NP, Kantarjian HM, Kim DW, Réa D, Dorlhiac-Llacer PE, Milone JH, Vela-Ojeda J, Silver RT, Khoury HJ, Charbonnier A, Khoroshko N, Paquette RL, Deininger M, Collins RH, Otero I, Hughes T, Bleickardt E, Strauss L, Francis S, Hochhaus A. Intermittent target inhibition with dasatinib 100 mg once daily preserves efficacy and improves tolerability in imatinib-resistant and-intolerant chronic-phase chronic myeloid leukemia. J Clin Oncol 2008; 26(19): 3204–3212
Shah NP, Kim DW, Kantarjian H, Rousselot P, Llacer PE, Enrico A, Vela-Ojeda J, Silver RT, Khoury HJ, Müller MC, Lambert A, Matloub Y, Hochhaus A. Potent, transient inhibition of BCR-ABL with dasatinib 100 mg daily achieves rapid and durable cytogenetic responses and high transformation-free survival rates in chronic phase chronic myeloid leukemia patients with resistance, suboptimal response or intolerance to imatinib. Haematologica 2010; 95(2): 232–240
Apperley JF, Cortes JE, Kim DW, Roy L, Roboz GJ, Rosti G, Bullorsky EO, Abruzzese E, Hochhaus A, Heim D, de Souza CA, Larson RA, Lipton JH, Khoury HJ, Kim HJ, Sillaber C, Hughes TP, Erben P, Van Tornout J, Stone RM. Dasatinib in the treatment of chronic myeloid leukemia in accelerated phase after imatinib failure: the START a trial. J Clin Oncol 2009; 27(21): 3472–3479
Hanfstein B, Müller MC, Hehlmann R, Erben P, Lauseker M, Fabarius A, Schnittger S, Haferlach C, Göhring G, Proetel U, Kolb HJ, Krause SW, Hofmann WK, Schubert J, Einsele H, Dengler J, Hänel M, Falge C, Kanz L, Neubauer A, Kneba M, Stegelmann F, Pfreundschuh M, Waller CF, Branford S, Hughes TP, Spiekermann K, Baerlocher GM, Pfirrmann M, Hasford J, Sauβele S, Hochhaus A; SAKK; German CML Study Group. Early molecular and cytogenetic response is predictive for long-term progression-free and overall survival in chronic myeloid leukemia (CML). Leukemia 2012; 26(9): 2096–2102
Kantarjian H, Cortes J, Kim DW, Dorlhiac-Llacer P, Pasquini R, DiPersio J, Müller MC, Radich JP, Khoury HJ, Khoroshko N, Bradley-Garelik MB, Zhu C, Tallman MS. Phase 3 study of dasatinib 140 mg once daily versus 70 mg twice daily in patients with chronic myeloid leukemia in accelerated phase resistant or intolerant to imatinib: 15-month median follow-up. Blood 2009; 113 (25): 6322–6329
Milojkovic D, Nicholson E, Apperley JF, Holyoake TL, Shepherd P, Drummond MW, Szydlo R, Bua M, Foroni L, Reid A, Khorashad JS, de Lavallade H, Rezvani K, Paliompeis C, Goldman JM, Marin D. Early prediction of success or failure of treatment with secondgeneration tyrosine kinase inhibitors in patients with chronic myeloid leukemia. Haematologica 2010; 95(2): 224–231
Marin D, Hedgley C, Clark RE, Apperley J, Foroni L, Milojkovic D, Pocock C, Goldman JM, O’Brien S. Predictive value of early molecular response in patients with chronic myeloid leukemia treated with first-line dasatinib. Blood 2012; 120(2): 291–294
Cortes JE, Saglio G, Kantarjian HM, Baccarani M, Mayer J, Boqué C, Shah NP, Chuah C, Casanova L, Bradley-Garelik B, Manos G, Hochhaus A. Final 5-year study results of DASISION: the dasatinib versus imatinib study in treatment-naïve chronic myeloid leukemia patients trial. J Clin Oncol 2016; 34(20): 2333–2340
Saglio G, Hochhaus A, Goh YT, Masszi T, Pasquini R, Maloisel F, Erben P, Cortes J, Paquette R, Bradley-Garelik MB, Zhu C, Dombret H. Dasatinib in imatinib-resistant or imatinib-intolerant chronic myeloid leukemia in blast phase after 2 years of follow-up in a phase 3 study: efficacy and tolerability of 140 milligrams once daily and 70 milligrams twice daily. Cancer 2010; 116(16): 3852–3861
Shah NP, Rousselot P, Schiffer C, Rea D, Cortes JE, Milone J, Mohamed H, Healey D, Kantarjian H, Hochhaus A, Saglio G. Dasatinib in imatinib-resistant or-intolerant chronic-phase, chronic myeloid leukemia patients: 7-year follow-up of study CA180-034. Am J Hematol 2016; 91(9): 869–874
Kim D, Goh HG, Kim SH, Cho BS, Kim DW. Long-term pattern of pleural effusion from chronic myeloid leukemia patients in secondline dasatinib therapy. Int J Hematol 2011; 94(4): 361–371
Steegmann JL, Baccarani M, Breccia M, Casado LF, García-Gutiérrez V, Hochhaus A, Kim DW, Kim TD, Khoury HJ, Le Coutre P, Mayer J, Milojkovic D, Porkka K, Rea D, Rosti G, Saussele S, Hehlmann R, Clark RE. European LeukemiaNet recommendations for the management and avoidance of adverse events of treatment in chronic myeloid leukaemia. Leukemia 2016; 30(8): 1648–1671
Schiffer CA, Cortes JE, Hochhaus A, Saglio G, le Coutre P, Porkka K, Mustjoki S, Mohamed H, Shah NP. Lymphocytosis after treatment with dasatinib in chronic myeloid leukemia: Effects on response and toxicity. Cancer 2016; 122(9): 1398–1407
Paydas S. Dasatinib, large granular lymphocytosis, and pleural effusion: useful or adverse effect? Crit Rev Oncol Hematol 2014; 89 (2): 242–247
Eskazan AE, Eyice D, Kurt EA, Elverdi T, Yalniz FF, Salihoglu A, Ar MC, Ongoren Aydin S, Baslar Z, Ferhanoglu B, Aydin Y, Tuzuner N, Ozbek U, Soysal T. Chronic myeloid leukemia patients who develop grade I/II pleural effusion under second-line dasatinib have better responses and outcomes than patients without pleural effusion. Leuk Res 2014; 38(7): 781–787
Acknowledgements
We thank the patients, their families, the investigators, and nurses, who participated in this trial. This research was funded by Bristol-Myers Squibb. Third-party writing assistance was funded by Bristol-Myers Squibb and provided by Manette Williams, PhD.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, X., Jiang, Q., Hu, J. et al. Four-year follow-up of patients with imatinib-resistant or intolerant chronic myeloid leukemia receiving dasatinib: efficacy and safety. Front. Med. 13, 344–353 (2019). https://doi.org/10.1007/s11684-018-0639-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11684-018-0639-7