Abstract
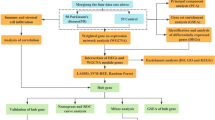
Parkinson’s disease (PD) is the most universal chronic degenerative neurological dyskinesia and an important threat to elderly health. At present, the researches of PD are mainly based on single-modal data analysis, while the fusion research of multi-modal data may provide more meaningful information in the aspect of comprehending the pathogenesis of PD. In this paper, 104 samples having resting functional magnetic resonance imaging (rfMRI) and gene data are from Parkinson’s Progression Markers Initiative (PPMI) and Alzheimer’s Disease Neuroimaging Initiative (ADNI) database to predict pathological brain areas and risk genes related to PD. In the experiment, Pearson correlation analysis is adopted to conduct fusion analysis from the data of genes and brain areas as multi-modal sample characteristics, and the clustering evolution random forest (CERF) method is applied to detect the discriminative genes and brain areas. The experimental results indicate that compared with several existing advanced methods, the CERF method can further improve the diagnosis of PD and healthy control, and can achieve a significant effect. More importantly, we find that there are some interesting associations between brain areas and genes in PD patients. Based on these associations, we notice that PD-related brain areas include angular gyrus, thalamus, posterior cingulate gyrus and paracentral lobule, and risk genes mainly include C6orf10, HLA-DPB1 and HLA-DOA. These discoveries have a significant contribution to the early prevention and clinical treatments of PD.







Similar content being viewed by others
References
Agliardi, C., Guerini, F. R., Zanzottera, M., Riboldazzi, G., Zangaglia, R., Sturchio, A., Casali, C., di Lorenzo, C., Minafra, B., Nemni, R., & Clerici, M. (2019). SNAP25 gene polymorphisms protect against Parkinson’s disease and modulate disease severity in patients. Molecular Neurobiology, 56(6), 4455–4463.
Akgun, A. (2012). A comparison of landslide susceptibility maps produced by logistic regression, multi-criteria decision, and likelihood ratio methods: A case study at İzmir, Turkey. Landslides, 9(1), 93–106.
Bologna, M., Leodori, G., Stirpe, P., Paparella, G., Colella, D., Belvisi, D., Fasano, A., Fabbrini, G., & Berardelli, A. (2016). Bradykinesia in early and advanced Parkinson's disease. Journal of the Neurological Sciences, 369, 286–291.
Chen, X., Wang, L., Qu, J., Guan, N.-N., & Li, J.-Q. (2018). Predicting miRNA–disease association based on inductive matrix completion. Bioinformatics, 34(24), 4256–4265.
Chen, X., Sun, Y.-Z., Guan, N.-N., Qu, J., Huang, Z.-A., Zhu, Z.-X., & Li, J. Q. (2019). Computational models for lncRNA function prediction and functional similarity calculation. Briefings in Functional Genomics, 18(1), 58–82.
Chouliaras, L., Pishva, E., Haapakoski, R., Zsoldos, E., Mahmood, A., Filippini, N., Burrage, J., Mill, J., Kivimäki, M., Lunnon, K., & Ebmeier, K. P. (2018). Peripheral DNA methylation, cognitive decline and brain aging: Pilot findings from the Whitehall II imaging study. Epigenomics, 10(5), 585–595.
Ciani, M., Benussi, L., Bonvicini, C., & Ghidoni, R. (2019). Genome wide association study and next generation sequencing: A glimmer of light towards new possible horizons in Frontotemporal dementia research. Frontiers in Neuroscience, 13, 506.
De Virgilio, A., Greco, A., Fabbrini, G., Inghilleri, M., Rizzo, M. I., Gallo, A., et al. (2016). Parkinson's disease: Autoimmunity and neuroinflammation. Autoimmunity Reviews, 15(10), 1005–1011.
Drucker, J., Sathian, K., Crosson, B., Krishnamurthy, V., McGregor, K. M., Bozzorg, A., et al. (2019). Internally guided lower limb movement recruits compensatory cerebellar activity in people with Parkinson’s disease. Frontiers in Neurology, 10, 537.
Du, L., Liu, K., Yao, X., Risacher, S. L., Han, J., Guo, L., et al. (2018). Fast multi-task SCCA learning with feature selection for multi-modal brain imaging genetics. In 2018 IEEE international conference on bioinformatics and biomedicine (BIBM) (pp. 356–361).
Du, L., Liu, K., Zhu, L., Yao, X., Risacher, S. L., Guo, L., et al. (2019). Identifying progressive imaging genetic patterns via multi-task sparse canonical correlation analysis: A longitudinal study of the ADNI cohort. Bioinformatics, 35(14), i474–i483.
Du, L., Liu, K., Yao, X., Risacher, S. L., Han, J., Saykin, A. J., et al. (2020). Detecting genetic associations with brain imaging phenotypes in Alzheimer’s disease via a novel structured SCCA approach. Medical Image Analysis, 61, 101656.
Falconi, A., Bonito-Oliva, A., Di Bartolomeo, M., Massimini, M., Fattapposta, F., Locuratolo, N., et al. (2019). On the role of adenosine A2A receptor gene transcriptional regulation in Parkinson’s disease. Frontiers in Neuroscience, 13, 683–692.
Fleming, S. M. (2017). Mechanisms of gene-environment interactions in Parkinson’s disease. Current environmental health reports, 4(2), 192–199.
Ghatak, S., Trudler, D., Dolatabadi, N., & Ambasudhan, R. (2018). Parkinson’s disease: What the model systems have taught us so far. Journal of Genetics, 97(3), 729–751.
Goldman, J., Fox, S., Isaacson, S., Fredericks, D., Trotter, J., Healy, K., et al. (2019). Examining Parkinson's disease psychosis treatment outcomes in the real world: Interim year 1 findings from the INSYTE observational study. The American Journal of Geriatric Psychiatry, 27(3), S180–S181.
Hao, X., J. Yan, X. Yao, S. L. Risacher, A. J. Saykin, D. Zhang, et al. (2016). Diagnosis-guided method for identifying multi-modality neuroimaging biomarkers associated with genetic risk factors in Alzheimer's disease. Biocomputing 2016: Proceedings of the Pacific Symposium, 108-119.
Huang, J., Zhu, Q., Hao, X., Shi, X., Gao, S., Xu, X., & Zhang, D. (2018). Identifying resting-state multifrequency biomarkers via tree-guided group sparse learning for schizophrenia classification. IEEE Journal of Biomedical and Health Informatics, 23(1), 342–350.
Jones-Davis, D. M., & Buckholtz, N. (2015). The impact of ADNI: What role do public-private partnerships have in pushing the boundaries of clinical and basic science research on Alzheimer's disease? Alzheimer's & dementia: the journal of the Alzheimer's Association, 11(7), 860–864.
Joshi, S., Davis, B., Jomier, M., & Gerig, G. (2004). Unbiased diffeomorphic atlas construction for computational anatomy. NeuroImage, 23, S151–S160.
Kaut, O., C. Mielacher, R. Hurlemann and U. Wüllner. (2020). Resting-state fMRI reveals increased functional connectivity in the cerebellum but decreased functional connectivity of the caudate nucleus in Parkinson’s disease. Neurological Research, 1-6.
Khawaldeh, S., Tinkhauser, G., Shah, S. A., Peterman, K., Debove, I., Nguyen, T. K., et al. (2020). Subthalamic nucleus activity dynamics and limb movement prediction in Parkinson’s disease. Brain, 143(2), 582–596.
Manes, J. L., Tjaden, K., Parrish, T., Simuni, T., Roberts, A., Greenlee, J. D., Corcos, D. M., & Kurani, A. S. (2018). Altered resting-state functional connectivity of the putamen and internal globus pallidus is related to speech impairment in Parkinson's disease. Brain and behavior, 8(9), e01073–e01092.
Marek, K., Chowdhury, S., Siderowf, A., Lasch, S., Coffey, C. S., Caspell-Garcia, C., Simuni, T., Jennings, D., Tanner, C. M., Trojanowski, J. Q., Shaw, L. M., Seibyl, J., Schuff, N., Singleton, A., Kieburtz, K., Toga, A. W., Mollenhauer, B., Galasko, D., Chahine, L. M., Weintraub, D., Foroud, T., Tosun-Turgut, D., Poston, K., Arnedo, V., Frasier, M., Sherer, T., the Parkinson's Progression Markers Initiative, Bressman, S., Merchant, M., Poewe, W., Kopil, C., Naito, A., Dorsey, R., Casaceli, C., Daegele, N., Albani, J., Uribe, L., Foster, E., Long, J., Seedorff, N., Crawford, K., Smith, D., Casalin, P., Malferrari, G., Halter, C., Heathers, L., Russell, D., Factor, S., Hogarth, P., Amara, A., Hauser, R., Jankovic, J., Stern, M., Hu, S. C., Todd, G., Saunders-Pullman, R., Richard, I., Saint-Hilaire, H., Seppi, K., Shill, H., Fernandez, H., Trenkwalder, C., Oertel, W., Berg, D., Brockman, K., Wurster, I., Rosenthal, L., Tai, Y., Pavese, N., Barone, P., Isaacson, S., Espay, A., Rowe, D., Brandabur, M., Tetrud, J., Liang, G., Iranzo, A., Tolosa, E., Marder, K., Sanchez, M., Stefanis, L., Marti, M., Martinez, J., Corvol, J. C., Assly, O., Brillman, S., Giladi, N., Smejdir, D., Pelaggi, J., Kausar, F., Rees, L., Sommerfield, B., Cresswell, M., Blair, C., Williams, K., Zimmerman, G., Guthrie, S., Rawlins, A., Donharl, L., Hunter, C., Tran, B., Darin, A., Venkov, H., Thomas, C. A., James, R., Heim, B., Deritis, P., Sprenger, F., Raymond, D., Willeke, D., Obradov, Z., Mule, J., Monahan, N., Gauss, K., Fontaine, D., Szpak, D., McCoy, A., Dunlop, B., Payne, L., Ainscough, S., Carvajal, L., Silverstein, R., Espay, K., Ranola, M., Rezola, E., Santana, H., Stamelou, M., Garrido, A., Carvalho, S., Kristiansen, G., Specketer, K., Mirlman, A., Facheris, M., Soares, H., Mintun, A., Cedarbaum, J., Taylor, P., Jennings, D., Slieker, L., McBride, B., Watson, C., Montagut, E., Sheikh, Z., Bingol, B., Forrat, R., Sardi, P., Fischer, T., Reith, D., Egebjerg, J., Larsen, L., Breysse, N., Meulien, D., Saba, B., Kiyasova, V., Min, C., McAvoy, T., Umek, R., Iredale, P., Edgerton, J., Santi, D., Czech, C., Boess, F., Sevigny, J., Kremer, T., Grachev, I., Merchant, K., Avbersek, A., Muglia, P., Stewart, A., Prashad, R., & Taucher, J. (2018). The Parkinson's progression markers initiative (PPMI)–establishing a PD biomarker cohort. Annals of clinical and translational neurology, 5(12), 1460–1477.
Martin, J. A., Zimmermann, N., Scheef, L., Jankowski, J., Paus, S., Schild, H. H., Klockgether, T., & Boecker, H. (2019). Disentangling motor planning and motor execution in unmedicated de novo Parkinson's disease patients: An fMRI study. NeuroImage: Clinical, 22, 101784.
Mihaescu, A. S., Masellis, M., Graff-Guerrero, A., Kim, J., Criaud, M., Cho, S. S., Ghadery, C., Valli, M., & Strafella, A. P. (2019). Brain degeneration in Parkinson’s disease patients with cognitive decline: A coordinate-based meta-analysis. Brain Imaging and Behavior, 13(4), 1021–1034.
Nalls, M. A., McLean, C. Y., Rick, J., Eberly, S., Hutten, S. J., Gwinn, K., et al. (2015). Diagnosis of Parkinson's disease on the basis of clinical and genetic classification: A population-based modelling study. The Lancet Neurology, 14(10), 1002–1009.
Owens-Walton, C., Jakabek, D., Power, B. D., Walterfang, M., Velakoulis, D., Van Westen, D., et al. (2019). Increased functional connectivity of thalamic subdivisions in patients with Parkinson’s disease. PLoS One, 14(9), e0222002.
Power, J. D., Plitt, M., Laumann, T. O., & Martin, A. (2017). Sources and implications of whole-brain fMRI signals in humans. Neuroimage, 146, 609–625.
Reynolds, R. H., Botía, J., Nalls, M. A., Hardy, J., Taliun, S. A. G., & Ryten, M. (2019). Moving beyond neurons: The role of cell type-specific gene regulation in Parkinson’s disease heritability. NPJ Parkinson's disease, 5(1), 1–14.
Rittman, T., Rubinov, M., Vértes, P. E., Patel, A. X., Ginestet, C. E., Ghosh, B. C., et al. (2016). Regional expression of the MAPT gene is associated with loss of hubs in brain networks and cognitive impairment in Parkinson disease and progressive supranuclear palsy. Neurobiology of Aging, 48, 153–160.
Robak, L. A., Jansen, I. E., Van Rooij, J., Uitterlinden, A. G., Kraaij, R., Jankovic, J., et al. (2017). Excessive burden of lysosomal storage disorder gene variants in Parkinson’s disease. Brain, 140(12), 3191–3203.
Santos-García, D., Mir, P., Cubo, E., Vela, L., Rodríguez-Oroz, M. C., Martí, M. J., et al. (2016). COPPADIS-2015 (COhort of patients with PArkinson’s DIsease in Spain, 2015), a global–clinical evaluations, serum biomarkers, genetic studies and neuroimaging–prospective, multicenter, non-interventional, long-term study on Parkinson’s disease progression. BMC Neurology, 16(1), 26–39.
Schober, P., Boer, C., & Schwarte, L. A. (2018). Correlation coefficients: Appropriate use and interpretation. Anesthesia & Analgesia, 126(5), 1763–1768.
Schwartz, F., Tahmasian, M., Maier, F., Rochhausen, L., Schnorrenberg, K. L., Samea, F., Seemiller, J., Zarei, M., Sorg, C., Drzezga, A., Timmermann, L., Meyer, T. D., van Eimeren, T., & Eggers, C. (2019). Overlapping and distinct neural metabolic patterns related to impulsivity and hypomania in Parkinson’s disease. Brain Imaging and Behavior, 13(1), 241–254.
Su, R., Liu, X., Wei, L., & Zou, Q. (2019). Deep-Resp-Forest: A deep forest model to predict anti-cancer drug response. Methods, 166, 91–102.
Tatura, R., Kraus, T., Giese, A., Arzberger, T., Buchholz, M., Höglinger, G., & Müller, U. (2016). Parkinson's disease: SNCA-, PARK2-, and LRRK2-targeting microRNAs elevated in cingulate gyrus. Parkinsonism & Related Disorders, 33, 115–121.
Thenganatt, M. A., & Jankovic, J. (2016). The relationship between essential tremor and Parkinson's disease. Parkinsonism & Related Disorders, 22, S162–S165.
Torigian, D. A., Kjær, A., Zaidi, H., & Alavi, A. (2016). PET/MR imaging: Clinical applications. PET clinics, 11(4), xi–xii.
Tysnes, O.-B., & Storstein, A. (2017). Epidemiology of Parkinson’s disease. Journal of Neural Transmission, 124(8), 901–905.
Wen, M. C., Chan, L., Tan, L., & Tan, E. (2016). Depression, anxiety, and apathy in Parkinson's disease: Insights from neuroimaging studies. European Journal of Neurology, 23(6), 1001–1019.
Wilson, H., Niccolini, F., Pellicano, C., & Politis, M. (2019). Cortical thinning across Parkinson's disease stages and clinical correlates. Journal of the Neurological Sciences, 398, 31–38.
Wu, C., Xu, G., Tsai, S.-Y. A., Freed, W. J., & Lee, C.-T. (2017). Transcriptional profiles of type 2 diabetes in human skeletal muscle reveal insulin resistance, metabolic defects, apoptosis, and molecular signatures of immune activation in response to infections. Biochemical and Biophysical Research Communications, 482(2), 282–288.
You, Z.-H., Zhou, M., Luo, X., & Li, S. (2016). Highly efficient framework for predicting interactions between proteins. IEEE transactions on cybernetics, 47(3), 731–743.
Younce, J. R., Campbell, M. C., Perlmutter, J. S., & Norris, S. A. (2019). Thalamic and ventricular volumes predict motor response to deep brain stimulation for Parkinson's disease. Parkinsonism & Related Disorders, 61, 64–69.
Acknowledgements
This work was supported by the Natural Science Foundation of Hunan Province, China (Grant No. 2020JJ4432, the Degree & Postgraduate Education Reform Project of Hunan Province (Grant No. 2019JGYB091), the Hunan Provincial Science and Technology Project Foundation (Grant No. 2018TP1018), the National Natural Science Foundation of China (Grant No. 61502167).
PPMI – a public-private partnership–funded by the Michael J. Fox Foundation for Parkinson’s Research and funding partners, including Abbvie, Avid Radiopharmaceuticals, Biogen, BioLegend, Bristol-Myers Squibb, General Electric Healthcare, Genentech, GlaxoSmithKline, Lilly, Lundbeck, Merck, Meso Scale Discovery, Pfizer, Piramal Imaging, Roche, Sanofi-Genzyme, Servier, Takeda, Teva and UCB.
Data collection and sharing for this project were also funded by the ADNI (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-20012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; BioClinica, Inc.; Biogen Idec Inc.; Bristol-Myers Squibb Company; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; F.Hoffmann-La Roche Ltd. and its affiliated company Genentech, Inc.; GE Healthcare; Innogenetics, N.V.; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Medpace, Inc.; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Synarc Inc.; and Takeda Pharmaceutical Company. Private sector contributions were facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no competing interest exists.
Informed consent
All the participants and their guardians have signed the informed consent.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI)- database (adni.loni.usc.edu) and the Parkinson’s Progression Markers Initiative (PPMI) database (www.ppmi-info.org/data). For up-to-date information of PPMI on the study, visit www.ppmi-info.org.As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf
Rights and permissions
About this article
Cite this article
Bi, Xa., Wu, H., Xie, Y. et al. The exploration of Parkinson’s disease: a multi-modal data analysis of resting functional magnetic resonance imaging and gene data. Brain Imaging and Behavior 15, 1986–1996 (2021). https://doi.org/10.1007/s11682-020-00392-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-020-00392-6