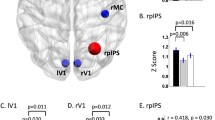
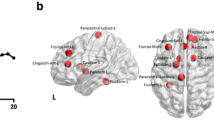
Abstract
Remitted late-life depression (rLLD) and amnestic mild cognitive impairment (aMCI) are both associated with a high risk of developing Alzheimer’s disease (AD). Neurodegeneration is considered to spread within pre-existing networks. To investigate whether, in the healthy brain, there was a pre-existing cross-network between the intrinsic networks that are vulnerable to rLLD and aMCI. We performed functional connectivity analyses based on brain areas with the greatest brain neuronal activity differences in 55 rLLD, 87 aMCI, and 114 healthy controls. Intrinsic networks that were differentially vulnerable to rLLD and aMCI converged onto the sensory-motor network (SMN) in the healthy brain. These regions in the SMN within the aMCI- and rLLD-vulnerable networks played different roles in the cognitive functions. This study identifies the SMN as a cross-network between rLLD- and aMCI-vulnerable networks. The common susceptibility of these diseases to AD is likely due to the breakdown of the cross-network. The results further suggest that interventions targeting the amelioration of sensory-motor deficits in the early course of disease in individuals with AD risk may enhance patient function as AD pathology progresses.




Similar content being viewed by others
References
Alalade, E., Denny, K., Potter, G., Steffens, D., & Wang, L. (2011). Altered cerebellar-cerebral functional connectivity in geriatric depression. PLoS One, 6(5), e20035.
Albers, M. W., Gilmore, G. C., Kaye, J., Murphy, C., Wingfield, A., Bennett, D. A., Boxer, A. L., Buchman, A. S., Cruickshanks, K. J., Devanand, D. P., Duffy, C. J., Gall, C. M., Gates, G. A., Granholm, A. C., Hensch, T., Holtzer, R., Hyman, B. T., Lin, F. R., McKee, A. C., Morris, J. C., Petersen, R. C., Silbert, L. C., Struble, R. G., Trojanowski, J. Q., Verghese, J., Wilson, D. A., Xu, S., & Zhang, L. I. (2015). At the interface of sensory and motor dysfunctions and Alzheimer's disease. Alzheimers Dement, 11(1), 70–98.
Alexopoulos, G. S. (2005). Depression in the elderly. Lancet, 365(9475), 1961–1970.
Alexopoulos, G. S., Meyers, B. S., et al. (1993). The course of geriatric depression with "reversible dementia": A controlled study. The American Journal of Psychiatry, 150(11), 1693–1699.
Apostolova, L. G., Dinov, I. D., Dutton, R. A., Hayashi, K. M., Toga, A. W., Cummings, J. L., & Thompson, P. M. (2006). 3D comparison of hippocampal atrophy in amnestic mild cognitive impairment and Alzheimer's disease. Brain, 129(Pt 11, 2867–2873.
Bai, F., Shu, N., Yuan, Y., Shi, Y., Yu, H., Wu, D., Wang, J., Xia, M., He, Y., & Zhang, Z. (2012). Topologically convergent and divergent structural connectivity patterns between patients with remitted geriatric depression and amnestic mild cognitive impairment. The Journal of Neuroscience, 32(12), 4307–4318.
Bhalla, R. K., Butters, M. A., Becker, J. T., Houck, P. R., Snitz, B. E., Lopez, O. L., Aizenstein, H. J., Raina, K. D., DeKosky, S. T., & Reynolds, C. F., III. (2009). Patterns of mild cognitive impairment after treatment of depression in the elderly. The American Journal of Geriatric Psychiatry, 17(4), 308–316.
Biswal, B., Yetkin, F. Z., et al. (1995). Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine, 34(4), 537–541.
Buckner, R. L., Sepulcre, J., Talukdar, T., Krienen, F. M., Liu, H., Hedden, T., Andrews-Hanna, J. R., Sperling, R. A., & Johnson, K. A. (2009). Cortical hubs revealed by intrinsic functional connectivity: Mapping, assessment of stability, and relation to Alzheimer's disease. The Journal of Neuroscience, 29(6), 1860–1873.
Buckner, R. L., Krienen, F. M., Castellanos, A., Diaz, J. C., & Yeo, B. T. T. (2011). The organization of the human cerebellum estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106(5), 2322–2345.
Buckner, R. L., Krienen, F. M., & Yeo, B. T. T. (2013). Opportunities and limitations of intrinsic functional connectivity MRI. Nature Neuroscience, 16(7), 832–837.
Butters, M. A., Klunk, W. E., Mathis, C. A., Price, J. C., Ziolko, S. K., Hoge, J. A., Tsopelas, N. D., Lopresti, B. J., Reynolds, C. F., III, DeKosky, S. T., & Meltzer, C. C. (2008). Imaging Alzheimer pathology in late-life depression with PET and Pittsburgh compound-B. Alzheimer Disease and Associated Disorders, 22(3), 261–268.
Byers, A. L., & Yaffe, K. (2011). Depression and risk of developing dementia. Nature Reviews. Neurology, 7(6), 323–331.
Byers, A. L., Covinsky, K. E., Barnes, D. E., & Yaffe, K. (2012). Dysthymia and depression increase risk of dementia and mortality among older veterans. The American Journal of Geriatric Psychiatry, 20(8), 664–672.
Cavanna, A. E., & Trimble, M. R. (2006). The precuneus: A review of its functional anatomy and behavioural correlates. Brain, 129(Pt 3, 564–583.
Chen, R., Hu, Z., Wei, L., Qin, X., McCracken, C., & Copeland, J. R. (2008). Severity of depression and risk for subsequent dementia: Cohort studies in China and the UK. The British Journal of Psychiatry, 193(5), 373–377.
Chen, J., Shu, H., et al. (2015a). The interaction of APOE genotype by age in amnestic mild cognitive impairment: A voxel-based morphometric study. Journal of Alzheimer's Disease, 43(2), 657–668.
Chen, J., Zhang, Z., & Li, S. (2015b). Can multi-modal neuroimaging evidence from hippocampus provide biomarkers for the progression of amnestic mild cognitive impairment? Neuroscience Bulletin, 31(1), 128–140.
Chen, J., Duan, X., Shu, H., Wang, Z., Long, Z., Liu, D., Liao, W., Shi, Y., Chen, H., & Zhang, Z. (2016a). Differential contributions of subregions of medial temporal lobe to memory system in amnestic mild cognitive impairment: Insights from fMRI study. Scientific Reports, 6, 26148.
Chen, J., Shu, H., Wang, Z., Liu, D., Shi, Y., Xu, L., & Zhang, Z. (2016b). Protective effect of APOE epsilon 2 on intrinsic functional connectivity of the entorhinal cortex is associated with better episodic memory in elderly individuals with risk factors for Alzheimer's disease. Oncotarget, 7(37), 58789–58801.
Chen, J., Shu, H., Wang, Z., Zhan, Y., Liu, D., Liao, W., Xu, L., Liu, Y., & Zhang, Z. (2016c). Convergent and divergent intranetwork and internetwork connectivity patterns in patients with remitted late-life depression and amnestic mild cognitive impairment. Cortex, 83, 194–211.
de Calignon, A., Polydoro, M., Suárez-Calvet, M., William, C., Adamowicz, D. H., Kopeikina, K. J., Pitstick, R., Sahara, N., Ashe, K. H., Carlson, G. A., Spires-Jones, T. L., & Hyman, B. T. (2012). Propagation of tau pathology in a model of early Alzheimer's disease. Neuron, 73(4), 685–697.
Drzezga, A., Becker, J. A., van Dijk, K. R. A., Sreenivasan, A., Talukdar, T., Sullivan, C., Schultz, A. P., Sepulcre, J., Putcha, D., Greve, D., Johnson, K. A., & Sperling, R. A. (2011). Neuronal dysfunction and disconnection of cortical hubs in non-demented subjects with elevated amyloid burden. Brain, 134(Pt 6, 1635–1646.
Fleisher, A. S., Sherzai, A., Taylor, C., Langbaum, J. B. S., Chen, K., & Buxton, R. B. (2009). Resting-state BOLD networks versus task-associated functional MRI for distinguishing Alzheimer's disease risk groups. Neuroimage, 47(4), 1678–1690.
Goncalves, S. I., de Munck, J. C., et al. (2006). Correlating the alpha rhythm to BOLD using simultaneous EEG/fMRI: Inter-subject variability. Neuroimage, 30(1), 203–213.
Gottwald, B., Mihajlovic, Z., Wilde, B., & Mehdorn, H. M. (2003). Does the cerebellum contribute to specific aspects of attention? Neuropsychologia, 41(11), 1452–1460.
Greicius, M. D., Krasnow, B., Reiss, A. L., & Menon, V. (2003). Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America, 100(1), 253–258.
Greicius, M. D., Srivastava, G., Reiss, A. L., & Menon, V. (2004). Default-mode network activity distinguishes Alzheimer's disease from healthy aging: Evidence from functional MRI. Proceedings of the National Academy of Sciences of the United States of America, 101(13), 4637–4642.
Habas, C., Kamdar, N., Nguyen, D., Prater, K., Beckmann, C. F., Menon, V., & Greicius, M. D. (2009). Distinct cerebellar contributions to intrinsic connectivity networks. The Journal of Neuroscience, 29(26), 8586–8594.
Huang, C., Wahlund, L. O., Almkvist, O., Elehu, D., Svensson, L., Jonsson, T., Winblad, B., & Julin, P. (2003). Voxel- and VOI-based analysis of SPECT CBF in relation to clinical and psychological heterogeneity of mild cognitive impairment. Neuroimage, 19(3), 1137–1144.
Huang, C., Eidelberg, D., Habeck, C., Moeller, J., Svensson, L., Tarabula, T., & Julin, P. (2007). Imaging markers of mild cognitive impairment: Multivariate analysis of CBF SPECT. Neurobiology of Aging, 28(7), 1062–1069.
Kemppainen, N. M., Aalto, S., Wilson, I. A., Nagren, K., Helin, S., Bruck, A., Oikonen, V., Kailajarvi, M., Scheinin, M., Viitanen, M., Parkkola, R., & Rinne, J. O. (2007). PET amyloid ligand [11C]PIB uptake is increased in mild cognitive impairment. Neurology, 68(19), 1603–1606.
Krienen, F. M., & Buckner, R. L. (2009). Segregated fronto-cerebellar circuits revealed by intrinsic functional connectivity. Cerebral Cortex, 19(10), 2485–2497.
Kumar, A., Bilker, W., Jin, Z., & Udupa, J. (2000). Atrophy and high intensity lesions: Complementary neurobiological mechanisms in late-life major depression. Neuropsychopharmacology, 22(3), 264–274.
La Joie, R., Landeau, B., et al. (2014). Intrinsic connectivity identifies the hippocampus as a main crossroad between Alzheimer's and semantic dementia-targeted networks. Neuron, 81(6), 1417–1428.
Landau, S. M., Harvey, D., Madison, C. M., Reiman, E. M., Foster, N. L., Aisen, P. S., Petersen, R. C., Shaw, L. M., Trojanowski, J. Q., Jack, C. R., Weiner, M. W., Jagust, W. J., & On behalf of the Alzheimer's Disease Neuroimaging Initiative. (2010). Comparing predictors of conversion and decline in mild cognitive impairment. Neurology, 75(3), 230–238.
Lehmann, M., Madison, C. M., Ghosh, P. M., Seeley, W. W., Mormino, E., Greicius, M. D., Gorno-Tempini, M. L., Kramer, J. H., Miller, B. L., Jagust, W. J., & Rabinovici, G. D. (2013). Intrinsic connectivity networks in healthy subjects explain clinical variability in Alzheimer's disease. Proceedings of the National Academy of Sciences of the United States of America, 110(28), 11606–11611.
Leichnetz, G. R. (2001). Connections of the medial posterior parietal cortex (area 7m) in the monkey. The Anatomical Record, 263(2), 215–236.
Liu, F., Hu, M., Wang, S., Guo, W., Zhao, J., Li, J., Xun, G., Long, Z., Zhang, J., Wang, Y., Zeng, L., Gao, Q., Wooderson, S. C., Chen, J., & Chen, H. (2012). Abnormal regional spontaneous neural activity in first-episode, treatment-naive patients with late-life depression: A resting-state fMRI study. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 39(2), 326–331.
Liu, Y., Yu, C., Zhang, X., Liu, J., Duan, Y., Alexander-Bloch, A. F., Liu, B., Jiang, T., & Bullmore, E. (2014). Impaired long distance functional connectivity and weighted network architecture in Alzheimer's disease. Cerebral Cortex, 24(6), 1422–1435.
Murphy, K., Birn, R. M., Handwerker, D. A., Jones, T. B., & Bandettini, P. A. (2009). The impact of global signal regression on resting state correlations: Are anti-correlated networks introduced? Neuroimage, 44(3), 893–905.
Myers, N., Pasquini, L., Göttler, J., Grimmer, T., Koch, K., Ortner, M., Neitzel, J., Mühlau, M., Förster, S., Kurz, A., Förstl, H., Zimmer, C., Wohlschläger, A. M., Riedl, V., Drzezga, A., & Sorg, C. (2014). Within-patient correspondence of amyloid-beta and intrinsic network connectivity in Alzheimer's disease. Brain, 137(Pt 7, 2052–2064.
Panza, F., Frisardi, V., Capurso, C., D'Introno, A., Colacicco, A. M., Imbimbo, B. P., Santamato, A., Vendemiale, G., Seripa, D., Pilotto, A., Capurso, A., & Solfrizzi, V. (2010). Late-life depression, mild cognitive impairment, and dementia: possible continuum? The American Journal of Geriatric Psychiatry, 18(2), 98–116.
Peraza, L. R., Colloby, S. J., et al. (2016). Regional functional synchronizations in dementia with Lewy bodies and Alzheimer's disease. International Psychogeriatrics, 1–9.
Petersen, R. C. (2011). Clinical practice. Mild cognitive impairment. The New England Journal of Medicine, 364(23), 2227–2234.
Petersen, R. C., & Negash, S. (2008). Mild cognitive impairment: An overview. CNS Spectrums, 13(1), 45–53.
Petersen, R. C., Smith, G. E., Waring, S. C., Ivnik, R. J., Tangalos, E. G., & Kokmen, E. (1999). Mild cognitive impairment: Clinical characterization and outcome. Archives of Neurology, 56(3), 303–308.
Power, J. D., Barnes, K. A., Snyder, A. Z., Schlaggar, B. L., & Petersen, S. E. (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage, 59(3), 2142–2154.
Raichle, M. E. (2011). The restless brain. Brain Connectivity, 1(1), 3–12.
Raichle, M. E., MacLeod, A. M., et al. (2001). A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America, 98(2), 676–682.
Saczynski, J. S., Beiser, A., Seshadri, S., Auerbach, S., Wolf, P. A., & Au, R. (2010). Depressive symptoms and risk of dementia: The Framingham heart study. Neurology, 75(1), 35–41.
Schmahmann JD, & Caplan D (2006) Cognition, emotion and the cerebellum. Brain 129(Pt 2): 290–292.
Seeley, W. W., Crawford, R. K., Zhou, J., Miller, B. L., & Greicius, M. D. (2009). Neurodegenerative diseases target large-scale human brain networks. Neuron, 62(1), 42–52.
Shirer, W. R., Ryali, S., Rykhlevskaia, E., Menon, V., & Greicius, M. D. (2012). Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cerebral Cortex, 22(1), 158–165.
Shmuel, A., & Leopold, D. A. (2008). Neuronal correlates of spontaneous fluctuations in fMRI signals in monkey visual cortex: Implications for functional connectivity at rest. Human Brain Mapping, 29(7), 751–761.
Shu, H., Shi, Y., Chen, G., Wang, Z., Liu, D., Yue, C., Ward, B. D., Li, W., Xu, Z., Chen, G., Guo, Q., Xu, J., Li, S. J., & Zhang, Z. (2016). Opposite neural trajectories of apolipoprotein E 4 and 2 alleles with aging associated with different risks of Alzheimer's disease. Cerebral Cortex, 26(4), 1421–1429.
Sjobeck, M., & Englund, E. (2001). Alzheimer's disease and the cerebellum: A morphologic study on neuronal and glial changes. Dementia and Geriatric Cognitive Disorders, 12(3), 211–218.
Smith, G. S., Kramer, E., Ma, Y., Kingsley, P., Dhawan, V., Chaly, T., & Eidelberg, D. (2009). The functional neuroanatomy of geriatric depression. International Journal of Geriatric Psychiatry, 24(8), 798–808.
Sorg, C., Riedl, V., Muhlau, M., Calhoun, V. D., Eichele, T., Laer, L., Drzezga, A., Forstl, H., Kurz, A., Zimmer, C., & Wohlschlager, A. M. (2007). Selective changes of resting-state networks in individuals at risk for Alzheimer's disease. Proceedings of the National Academy of Sciences of the United States of America, 104(47), 18760–18765.
Steffens, D. C., & Potter, G. G. (2008). Geriatric depression and cognitive impairment. Psychological Medicine, 38(2), 163–175.
Steffens, D. C., Otey, E., Alexopoulos, G. S., Butters, M. A., Cuthbert, B., Ganguli, M., Geda, Y. E., Hendrie, H. C., Krishnan, R. R., Kumar, A., Lopez, O. L., Lyketsos, C. G., Mast, B. T., Morris, J. C., Norton, M. C., Peavy, G. M., Petersen, R. C., Reynolds, C. F., Salloway, S., Welsh-Bohmer, K. A., & Yesavage, J. (2006). Perspectives on depression, mild cognitive impairment, and cognitive decline. Archives of General Psychiatry, 63(2), 130–138.
Stoodley, C. J., Valera, E. M., & Schmahmann, J. D. (2010). An fMRI study of intra-individual functional topography in the human cerebellum. Behavioural Neurology, 23(1–2), 65–79.
Tadayonnejad, R., & Ajilore, O. (2014). Brain network dysfunction in late-life depression: A literature review. Journal of Geriatric Psychiatry and Neurology, 27(1), 5–12.
Thal, D. R., Rub, U., et al. (2002). Phases of a beta-deposition in the human brain and its relevance for the development of AD. Neurology, 58(12), 1791–1800.
Thomann, P. A., Schlafer, C., et al. (2008). The cerebellum in mild cognitive impairment and Alzheimer's disease - a structural MRI study. Journal of Psychiatric Research, 42(14), 1198–1202.
Van Dijk, K. R., Sabuncu, M. R., et al. (2012). The influence of head motion on intrinsic functional connectivity MRI. Neuroimage, 59(1), 431–438.
Videbech, P., & Ravnkilde, B. (2004). Hippocampal volume and depression: A meta-analysis of MRI studies. The American Journal of Psychiatry, 161(11), 1957–1966.
Wang, Z., Yuan, Y., Bai, F., You, J., Li, L., & Zhang, Z. (2012). Abnormal default-mode network in angiotensin converting enzyme D allele carriers with remitted geriatric depression. Behavioural Brain Research, 230(2), 325–332.
Weissenbacher, A., Kasess, C., Gerstl, F., Lanzenberger, R., Moser, E., & Windischberger, C. (2009). Correlations and anticorrelations in resting-state functional connectivity MRI: A quantitative comparison of preprocessing strategies. Neuroimage, 47(4), 1408–1416.
Winblad, B., Palmer, K., Kivipelto, M., Jelic, V., Fratiglioni, L., Wahlund, L. O., Nordberg, A., Backman, L., Albert, M., Almkvist, O., Arai, H., Basun, H., Blennow, K., de Leon, M., DeCarli, C., Erkinjuntti, T., Giacobini, E., Graff, C., Hardy, J., Jack, C., Jorm, A., Ritchie, K., van Duijn, C., Visser, P., & Petersen, R. C. (2004). Mild cognitive impairment--beyond controversies, towards a consensus: Report of the international working group on mild cognitive impairment. Journal of Internal Medicine, 256(3), 240–246.
Zang, Y. F., He, Y., Zhu, C. Z., Cao, Q. J., Sui, M. Q., Liang, M., Tian, L. X., Jiang, T. Z., & Wang, Y. F. (2007). Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev, 29(2), 83–91.
Zhou, J., Gennatas, E. D., Kramer, J. H., Miller, B. L., & Seeley, W. W. (2012). Predicting regional neurodegeneration from the healthy brain functional connectome. Neuron, 73(6), 1216–1227.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Nos. 81420108012, 81671046, 81871438, 81571062 and 81701675), the Disciplinary group of Psychology and Neuroscience, Xinxiang Medical University (No. 2016PNKFKT-01), the Strategic Priority Research Program (B) of Chinese Academy of Sciences (Grant No. XDB32020200), the Key Project supported by Medical Science and technology development Foundation, Nanjing Department of Health (No. JQX18005), the Cooperative Research Project of Southeast University-Nanjing Medical University (No. 2018DN0031), and the Key Research and Development Plan (Social Development) Project of Jiangsu Province (No. BE2018608). The authors thank Xiaofa Huang and Hong Zhu for their help with the acquisition of the behavioral data and for taking care of the clinical data in this study.
Author Contributors
Author JC undertook the data analysis and wrote the manuscript. Authors HS, ZW, DL acquired the data. Authors CJ, YL, and ZJZ designed the study. Authors JC, YZ, and YL supervised the data analysis. Author YL and ZJZ provided infrastructure. All authors contributed to and have approved the final manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (Nos. 81420108012, 81671046, 81871438, 81571062 and 81701675), the Disciplinary group of Psychology and Neuroscience, Xinxiang Medical University (No. 2016PNKFKT-01), the Strategic Priority Research Program (B) of Chinese Academy of Sciences (Grant No. XDB32020200), the Key Project supported by Medical Science and technology development Foundation, Nanjing Department of Health (No. JQX18005), the Cooperative Research Project of Southeast University-Nanjing Medical University (No. 2018DN0031), and the Key Research and Development Plan (Social Development) Project of Jiangsu Province (No. BE2018608).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1801 kb)
Rights and permissions
About this article
Cite this article
Chen, J., Shu, H., Wang, Z. et al. Intrinsic connectivity identifies the sensory-motor network as a main cross-network between remitted late-life depression- and amnestic mild cognitive impairment-targeted networks. Brain Imaging and Behavior 14, 1130–1142 (2020). https://doi.org/10.1007/s11682-019-00098-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-019-00098-4