Abstract
Climate change is causing more frequent and severe climatic events, such as extreme heat and co-occurring drought, potentially accelerating tree mortality. Which tree species will cope better with those extreme events is still being researched. This study focuses on heat as a physiological stress factor and interspecific variation of thermal tolerance and sensitivity traits in 15 temperate coniferous and broad-leaved tree species. We investigate (1) whether thermal tolerance and sensitivity traits correlate with a drought-related physiological trait, particularly the leaf turgor loss point (πtlp, wilting point), and (2) how thermal tolerance and sensitivity traits co-vary within different tree-functional types classified by morphological and physiological traits of the leaf, i.e., leaf mass per area (LMA) and percentage loss of area (PLA). The study was carried out in the Traunstein Forest Dynamics Plot of the ForestGEO network in Germany. The temperature response of the maximum quantum yield of photosystem II (Fv/Fm) on leaf discs was determined, from which various physiological leaf traits were estimated, one of which is the breaking point temperature (T5), the temperature at which Fv/Fm declines by 5%. Additionally, the temperature of 50% (T50) and 95% (T95) decline in Fv/Fm was evaluated. The decline width between T50 and T5 (DWT50−T5) was taken as an indicator of the species’ thermal sensitivity. The breaking point temperature ranged from 35.4 ± 3.0 to 47.9 ± 3.9 °C among the investigated tree species and T50 ranged between 46.1 ± 0.4 and 53.6 ± 0.7 °C. A large interspecific variation of thermal tolerance and sensitivity was found. European ash (Fraxinus excelsior L.) was the most heat-sensitive species, while Wild cherry (Prunus avium L.) was the least heat-sensitive species. Species with a more negative πtlp tended to have a higher breaking point temperature than species with a less negative πtlp. A lower thermal sensitivity characterized species with a higher LMA, and high PLA was found in species with low thermal sensitivity. Accordingly, species with thicker and tougher leaves have lower thermal sensitivity which coincides with a lower wilting point. We conclude that species that develop drought-adapted foliage can cope better with heat stress. Further, they might be able to maintain transpirational cooling during combined heat and drought stress, which could lessen their mortality risk during climatic extremes.
Similar content being viewed by others
Introduction
In 2022, Europe was struck by record-breaking climate extremes, with the hottest heatwave and the longest-lasting drought spell in history (European Union, Copernicus Climate Change Service 2022). 2022 has only been the tip of the iceberg, as the frequency and intensity of hot and dry summers have increased dramatically in recent years (Liao et al. 2020; Rousi et al. 2022). In the face of this rapidly changing climate, it is expected that climatic thresholds of locally adapted forests will be exceeded and are likely to overcome critical forest resilience and resistance thresholds, leading to accelerating forest mortality (Alizadeh et al. 2020; Senf et al. 2022). The effect of surpassed climatic thresholds can already be observed in many forests in Central Europe as large-scale and climate-driven forest mortality events have drastically augmented over the last decades (Senf et al. 2018). In the literature, climate-induced drought is underlined as the main driver for high forest mortality (Senf et al. 2020). However, heat or the combination of heat and drought as possible drivers have received much less attention (Hammond et al. 2022; Salomón et al. 2022). Central European forests may be particularly vulnerable as a few tree species dominate them, i.e., Scots pine (Pinus sylvestris L.), Norway spruce (Picea abies (L.) H. Karst.), European beech (Fagus sylvatica L.) and Pedunculate oak (Quercus robur L.) are particularly susceptible to heat and drought stress (Buras and Menzel 2018; Leuschner 2020; Bose et al. 2021; Húdoková et al. 2022). There is an urgent need to improve our understanding of the thermal tolerance and temperature sensitivity of tree species in the mid-latitude. Species in those areas tend to have the “narrowest thermal safety margins“ and are exposed to a high risk of more frequent heatwaves (O’Sullivan et al. 2017). However, little is known about the susceptibility to heat stress of the species forming these forests. Even less is known about which species could substitute those main species, particularly in managed forest systems and in the perspective of heat or combined heat and drought stress.
To understand the extent to which tree species can cope with heat or hotter drought events, species-specific responses can be quantified using leaf functional, morphological, and physiological traits related to heat and drought stress (Reich et al. 2007; Bussotti 2008). For example, Scoffoni et al. (2014) show that the two functional and morphological traits, leaf mass per area (LMA) and percentage loss of area (PLA), are good predictors for the drought tolerance of leaves and correlate with leaf hydraulic traits, such as the turgor loss point (πtlp). Further, LMA, defined as the ratio between leaf mass and leaf area in g m−2, is a key trait in measuring leaf sclerophylly (Reich et al. 1992; Niinemets 2001; Wright et al. 2004; Ordoñez et al. 2009; Kattge et al. 2011; Kikuzawa et al. 2013), which in turn is linked to xeromorphy via the implication of equivalent leaf anatomical and morphological adaptations to drought (Medina et al. 1990). Xeromorphic leaves typically have, e.g., a smaller leaf area with a multi-layered epidermis, thicker cuticle, compactly arranged mesophyll with low air spaces, and high stomatal density to reduce tissue desiccation during periods of high evaporative demand (Bussotti 2008; Binks et al. 2016; La Riva et al. 2016). Xeromorphic leaves are heavier compared to mesomorphic leaves. According to Schimper et al. (1903), evergreen xeromorphic woody plants which are local to Mediterranean areas with prolonged summer drought, possess sclerophyllous leaves. Moreover, xeromorphism and sclerophylly are combined by implying a higher LMA (Oppenheimer 1960). Although xeromorphism per se is not an adaptation to high summer temperatures, LMA is known to affect leaf heat capacity and rates of change in leaf temperature (Groom et al. 2004). Consequently, LMA is suitable as a key trait to quantify the effects of thermal and co-occurring drought stress on leaf morphology and their ability to withstand them. A higher LMA suggests a higher structural investment in leaf tissues, thus providing longer longevity but lower leaf nitrogen concentrations, growth, and photosynthetic rates (Wright et al. 2002). These observations indicate that species with a higher structural investment in leaf tissue, e.g., with thicker cell walls, might be able to prevent shrinkage of leaf surface area during periods with limited water ability and high evaporative demand. They may also sustain water transport capacity, showing a smaller PLA (Nardini 2022). However, LMA and traits moderating leaf thermal properties are positively correlated (Greenwood et al. 2017).
Leaf thermal properties can be evaluated by assessing the temperature dependence of the photochemical efficiency of the photosystem II (PSII). The PSII represents the most heat-sensitive component of the photosynthetic system (Berry and Bjorkman 1980). When critical temperature thresholds are exceeded, permanent impairment of PSII function occurs, and photosynthetic capacity can be severely limited. The effect of heat stress on the photochemical efficiency of PSII can be quantified by the ratio between variable and maximum chlorophyll fluorescence (Fv/Fm). Fv/Fm can be used to calculate thermal tolerance traits such as T5, T50, and T95. T5 represents the breakpoint temperature at which Fv/Fm drops below 95% of the maximum yield of PSII. T50 defines the temperature associated with a 50% decline in Fv/Fm (Krause et al. 2010), whereas T95 defines the temperature at which Fv/Fm is reduced by 95%. According to Tiwari et al. (2021), T95 also functions as a threshold value above which PS II functions are effectively lost. Additionally, they suggest determining the thermal sensitivity of species by the decline width (DW), defined as the width of the decline of various temperature levels-induced decrease in the photosynthetic conversion rate of PSII. In species considered heat tolerant, maximum PSII quantum yield (QY) is maintained at high temperatures (high T5). However, it is characterized by a rapid decline if the breaking point temperature is surpassed. Conversely, heat-sensitive plants are characterized by low T5 values, and the decline of PSII in QY occurs gradually over a wide temperature range.
Most studies focusing on the relationship between functional and morphological traits and leaf thermal properties have been conducted in predominantly seasonal dry tropical forests (Sastry and Barua 2017; Sastry et al. 2018) or desert ecosystems (Knight and Ackerly 2003; Curtis et al. 2014). Only a few studies target the interplay between thermal properties and leaf traits in boreal and temperate forests (Ruiz-Pérez et al. 2019; Kitudom et al. 2022). In this study, we wanted to test whether leaf thermal properties are related to functional, morphological, and physiological traits, namely LMA, PLA, and πtlp, in a cool temperate montane wet forest. Therefore, we assessed the sensitivity of the PSII to temperature changes for 15 tree species representing a relatively wide range of the regional species pool in a Central European Forest. We investigated (1) whether leaf thermal tolerance and sensitivity correlate with a drought-related physiological trait and (2) if thermal tolerance and sensitivity vary within different tree-functional types classified by leaf morphological and physiological traits. The study was carried out in the ForestGEO plot in Traunstein, Germany.
Material and methods
Study site and tree species
The botanical material from 15 temperate tree species was collected at the 25 ha Traunstein ForestGEO plot, which is part of the Forest Global Earth Observatory network (ForestGEO) (Davies et al. 2021), Germany. The study site is located at the former hermitage in Froschham, approximately 10 km outside of Traunstein, in the southeastern part of Germany (47.935000°N, 12.666400°E). The forest can be classified as a cool temperate montane wet forest (Holdridge 1947). The plot lies in the vicinity of the northern Alps at an altitude of 590 m. a.s.l. The mean annual temperature is 9.1 °C, and the mean annual precipitation is 1060 mm (Fig. 1). Due to the particular geographical condition, rainfall is not expected to decline with climate change significantly. However, the number of hot days has increased over the last four decades (Fig. 2), and there is a high risk of future heat waves (Senf and Lakes 2011). In total, 25 different tree species have been identified on the plot, whereby Norway spruce is the dominant, representing 50% of the basal area, followed by Sycamore maple (Acer pseudoplatanus L.), European hornbeam (Carpinus betulus L.), European beech (Fagus sylvatica L.), and European larch (Larix decidua, Mill.) (Giammarchi et al. 2017). At the former hermitage, an arboretum has been established, which offers to investigate a variety of non-native tree species to test their potential as alternative species to climate change. The Traunstein ForestGEO plot is currently subject to actively managed forest plots and is thus representative of the vast majority of forests in Central Europe. The eastern part of the area is dominated by a homogeneous, mostly monospecific structure consisting of Norway spruce and a grid of Sycamore maple to increase the wind resistance of the forest. Forest management aims to transform those monospecific stands into uneven-aged mixed-species stands. The western part of the plot is dominated by multi-layered mixed stands where the transformation into complex stand structures has been successfully taking place for decades (ForestGEO 2017).
Sample collection
With our study design, we wanted to screen for the highest possible variation in tree-functional types. Therefore, we chose 15 tree species growing at the Traunstein ForestGEO site. Of those 15 tree species (12 broadleaved and three coniferous tree species), 14 are native to Central Europe, and one is an introduced tree species (a complete list of species is provided in Table 1). The introduced tree species, namely the bald cypress (Taxodium distichum (L.) Rich.), was chosen due to its leaf morphology. The bald cypress is a deciduous conifer with needle-like leaves arranged on little branchlets characterized by an LMA in the range of the local broad-leaved species (Table 1). In contrast, the two native conifers, Silver fir and Norway spruce have typical needle-like leaves with a tenfold higher LMA than the broadleaved tree species. We expected a mismatch if the LMA of the two conifers was pooled with the broadleaved species. Therefore, we wanted to test whether tendencies between conifers and broadleaves align if species with a similar range of LMA were chosen.
We chose four mature individuals for each tree species with a minimum diameter of 25 cm at breast height. We collected most of the samples from tree individuals growing at the forest edge where branches could be sampled with a 10 m long pruner. We assumed that intraspecific trait variation was lower than interspecific trait variation in sun-exposed leaves. Therefore, all collected branches were fully sun-exposed and not shaded within the tree crown or the surrounding forest canopy. One sun-exposed branch was collected per tree. Branches were between 50 and 100 cm long. The freshly cut branches were placed in opaque plastic bags and immediately transported to the laboratory for further processing as fast as possible (< 30 min). The branches were re-cut under water at least two nodes distal to the initial cutting to avoid embolisms and placed into water-filled buckets covered with an opaque plastic bag to ensure a moisture-saturated atmosphere. The branches were left to rehydrate overnight (> 8 h).
Thermal tolerance and sensitivity measurements
We selected eight healthy and fully functional leaves per tree from the distal end of a given branch to be assigned to one of eight temperature treatments. Leaf disks (diameter 2 cm), excluding the midrib and mayor veins, were cut out of the leaves. To ensure the health of the chosen leaves, photosynthetic efficiency was measured using a chlorophyll fluorometer (MINI-PAM, Walz, Effeltrich, Germany) on the leaf disks that had been dark acclimated. We used the recommended setting by Krause et al. (2006) to measure Fv/Fm. Briefly, the “measuring light burst” mode was used to receive an exact measurement of the initial fluorescence (Fo). Therefore, the measuring light frequency was set low (0.6 kHz). A 1.0 s duration saturating light pulse was applied to record Fm. A leaf with an Fv/Fm above 0.75 was healthy. The thermal dependency of Fv/Fm was assessed following the protocol of Krause et al. (2010). The leaf disks were wrapped in moist tissue and placed into water-tight Whirl-Pack bags. The bags were then immersed in a temperature-controlled water bath and exposed to treatment temperatures for 30 min. We used Sous-Vide precision cookers for precise temperature regulation (25 °C to 60 °C), increasing the temperature in steps of 5 °C (Kunert and Hajek 2022). Afterward, the leaves were allowed to rest for several hours at a low light level, and dark acclimate for at least 30 min before the final Fv/Fm measurement.
Determination of turgor loss point
Two leaf samples were collected from three individuals of each species for the determination of water potential at the turgor loss point using vapor pressure osmometry the following day. We started by cutting two discs per leaf with a 4-mm cork borer and wrapping them into aluminum foil. Then, the samples were submerged in liquid nitrogen (LN2) for at least two minutes. After the leaf discs were deep frozen to rupture cell walls, they were pricked about 20 times with a dissecting needle and placed in the measuring chamber (10 μL) of the vapor pressure osmometer. When the equilibrium was established, the osmolarity values given by the device were used to calculate the osmotic potential at full hydration and the turgor loss point (πtlp) using the standard conversion factors established by Bartlett et al. (2012).
Estimation of functional leaf traits
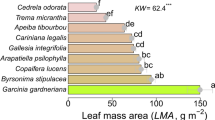
For estimating functional leaf traits such as LMA (g m−2) and PLA (%), we collected fully expanded leaves of three individuals per species without any symptoms of disease or damage. For the measurement of the LMA, midrib and all veins are considered part of the leaf. We collected and cut the branches with leaves still attached as described above. Before measuring, each leaf was freshly cut from the stem, and the petioles were removed. Next, the freshly cut leaves were scanned with a flatbed scanner (OpticSlim 2610 Plus Scanner, Plustek, Taipei, Taiwan) and stored as image files. The leaf area was calculated from the scanned images using a self-developed R-Script. Subsequently, the leaves were dried in an oven at 60 °C for at least two days. The dried leaves were scanned again, and dry weight was measured. LMA was calculated as the ratio of leaf dry mass to leaf area (g m−2), and the PLA was calculated as the ratio of leaf area between dried leaves and freshly cut leaves (%) (Cornelissen et al. 2003).
Data processing
For the calculation of the Fv/Fm response, a log-logistic curve according to Kunert et al. (2021a) was used, Eq. 1:
where T describes the temperature and T50 describes the temperature above which Fv/Fm corresponds to 50% of the maximum. The slope of the curve at T = T50 is described with b, while Fv/Fm of the lower plateau is described with c and the Fv/Fm of the higher plateau with d. If an asymmetric curve shape described the thermal response of a species, the following extension of Eq. 1 was used. This allows curvature differences before and after T = T50, Eq. 2:
where, b1 and b2 describe differences in curvature around T50. To find the best fitting function, the ‘modelFit’ function from the ‘drc’ package in R was used (Ritz et al. 2015). Using Akaike’s (1974) information criterion based on the selection of the model with the smallest AIC describing the best fit, we decided whether to use Eq. (1) or Eq. (2). Six different values of the fitted curves were calculated to assess if differences in the thermal responses of the species existed. With the ED function, we could calculate T5, T50, and T95. T5 describes the temperature at which the decline is 5%, T50 with a decline of 50%, and T95 with 95% of the maximum change (d − c). Data analysis was performed using the R program, version 4.2.1 (R Core Team 2022).
Statistical analysis
Pearson’s product-moment correlation coefficient was utilized to measure the bivariate relationships between T5 and DWT95−T5, T50−5, T95−T50 in the tree species sampled in Traunstein. In addition, correlations were analyzed between TLP, thermal sensitivity, and thermal tolerance values among the tree species sampled in Traunstein supported by additional data from Vienna. LMA and PLA were also evaluated for their correlation with thermal sensitivity and tolerance values of the tree species sampled in Traunstein. The assumptions of normality and homogeneity of variance were tested using Shapiro–Wilk and Levene’s test, respectively. The data analysis was conducted using the R software package (R Core Team 2022).
Results
T5 averaged 41.7 ± 1.5 °C across all 15 measured species. The highest T5 was observed in Wild cherry (Prunus avium L.) at 47.9 ± 3.9 °C and lowest in European ash at 35.4 ± 1.4 °C. Between species, variation of T5 within the genus was higher for Acer with 38.2 ± 1.2 to 41.4 ± 1.4 °C than for Salix ranging from 42.5 ± 0.5 to 42.5 ± 0.8 °C. T50 values were, on average, at 48.7 ± 0.4 °C with Norway maple (Acer platanoides L.) having the highest T50 (53.6 ± 0.7 °C). The lowest T50 values have been found in Silver fir (45.2 ± 0.5 °C) and European ash (46.1 ± 0.37 °C). For T95 an average temperature of 52.7 ± 1.3 °C has been found. The highest T95 was measured for European ash (55.3 ± 0.7 °C) and lowest for Silver fir (47.9 ± 6.6 °C). As for the width of decline between T50 and T5, the average was 7.0 °C. The highest DWT50−T5 was found in Norway maple at 15.5 °C and lowest in Wild cherry at 1.6 °C.
Regarding the πtlp, an average πtlp with − 2.01 ± 0.04 kPa, the most negative πtlp for European beech (− 2.58 ± 0.04 kPa), and least negative for Goat willow (Salix caprea L.) (− 1.35 ± 0.03 kPa) were observed. LMA was highest in Norway spruce and the lowest in Norway maple. Leaves of European alder (Alnus glutinosa (L.) in Gaertn.) were characterized by the highest reduction in leaf area (PLA) (36.44%) and Silver fir (6.69%) by the lowest reduction (Fig. 3).
For the T5 values, a significant negative correlation with the DWT50−T5 (R2 = 0.947, P < 0.001) were observed. Species with lower T5 had a wider decline width from T50 − T5 (Fig. 4). The data set was extended by 12 species from Kunert and Hajek (2022) which aligned with these relationships, and species with a higher T5 showed a steeper and more sudden decline in Fv/Fm with a narrow DWT50−T5.
Relationships between the breakpoint temperature (T5) and the decline width (DW). Three different temperature windows are present. a Shows when Fv/Fm declines from 95 to 5% of the maximum Fv/Fm level (DWT95−T5, broad-leaved trees (Traunstein): y = − 0.90x + 51.18, R2 = 0.37, P < 0.05), b the Fv/Fm decline from 50 to 5% of the maximum Fv/Fm level (DWT50−T5, broad-leaved trees (Traunstein): y = − 1.08x + 54.15, R2 = 0,51, P < 0.05), and c Fv/Fm declines from 95 to 50% of the maximum Fv/Fm level (DWT95−T50, broad-leaved trees (Traunstein): y = 0.18x − 2.97, R2 = 0.04, P > 0.1)
Significant correlations were reported between πtlp and T50 (R2 = 0.296, P < 0.05) and T95 values (R2 = 0.229, P < 0.05), but T5 values did not correlate significantly (R2 = 0.04, P > 0.05). Tree species characterized by a more negative πtlp showed higher T50 and T95 values. The additional data set aligned with the mentioned relationships. A trend could be observed showing that species with a higher T50 (R2 = 0.296, P < 0.05) and T95 (R2 = 0.229, P < 0.05) were characterized by a more negative πtlp (Fig. 5). No significant statistical relationship was observed between πtlp and thermal sensitivity traits (DWT95−T5, T50−T5, T95−T50) (Fig. 6).
Relationships between thermal tolerance traits and leaf turgor loss point (πtlp). a The breaking point temperature (T5, broad-leaved trees (Traunstein, Vienna): y = -1.72x + 38.79, R2 = 0.03, P > 0.1, b the temperature of 50% decline of Fv/Fm (T50, broad-leaved trees (Traunstein, Vienna): y = -5.09 + 40.22, R2 = 0.296, P < 0.05, c the temperature of 95% decline of Fv/Fm (T95 broad-leaved trees (Traunstein, Vienna): y = − 5.11 + 44.72, R2 = 0.229, P < 0.05
Relationships between the osmotic potential at turgor loss (πtlp) and the decline width (DW). Three different temperature windows are present. a Shows when Fv/Fm declines from 95 to 5% of the maximum Fv/Fm level (DWT95−T5, broad-leaved trees (Traunstein, Vienna): y = -3.39x + 5.93, R2 = 0.06, P > 0.1), b the Fv/Fm decline from 50 to 5% of the maximum Fv/Fm level (DWT50−T5, broad-leaved trees (Traunstein, Vienna): y = − 3.5x + 1.06, R2 = 0,07, P > 0.1), and c Fv/Fm declines from 95 to 50% of the maximum Fv/Fm level (DWT95−T50, broad-leaved trees (Traunstein, Vienna): y = 0.13x + 4.91, R2 = 2.53e−4, P > 0.1). O means broad-leaves species (Vienna), • means broad-leaved species (Traunstein), Δ conifers (Traunstein)
An absence of significant correlation was discovered between LMA and T5 (R2 = 0.06, P > 0.1) in broadleaved trees during the investigation of the relationship between thermotolerance traits and LMA. However, a significant correlation was observed between LMA and T50 (R2 = 0.46, P < 0.05), indicating that broad-leaved species with higher LMA have a lower T50 value. T95 was found to significantly correlate with LMA values with R2 = 0.44, P < 0.05 (Fig. 7). Furthermore, significant correlations were observed for LMA with DWT95−T5 (R2 = 0.28, P < 0.1) and DWT50−T5 (R2 = 0.34, P < 0.05), showing that species with higher LMA have a smaller DW. Thus, species with smaller LMA have a higher sensitivity to heat (Fig. 8).
Relationships between thermal tolerance traits and leaf mass area (LMA). a The breaking point temperature (T5, broad-leaved trees (Traunstein): y = 0.54x -0.26, R2 = 0.06, P > 0.1, b the temperature of 50% decline of Fv/Fm (T50, broad-leaved trees (Traunstein): y = − 2.04 + 122.31, R2 = 0.46, P < 0.05, c the temperature of 95% decline of Fv/Fm (T95 broad-leaved trees (Traunstein): y = − 2.23 + 140.88, R2 = 0.44, P < 0.05). • means broad-leaved species (Traunstein), Δ conifers (Traunstein)
Relationships between the leaf mass per area (LMA) and the decline width (DW). Three different temperature windows are present. a Shows when Fv/Fm declines from 95 to 5% of the maximum Fv/Fm level (DWT95−T5, broad-leaved trees (Traunstein): y = -0.91x + 32.82, R2 = 0.280, P > 0.1), b the Fv/Fm decline from 50 to 5% of the maximum Fv/Fm level (DWT50−T5, broad-leaved trees (Traunstein): y = -0.99x + 29.79, R2 = 0,34, P > 0.1), and (c) Fv/Fm declines from 95 to 50% of the maximum Fv/Fm m level (DWT95−T50, broad-leaved trees (Traunstein): y = 0.32x + 20.97, R2 = 0.01, P > 0.1). • means broad-leaved species (Traunstein), Δ conifers (Traunstein)
In addition, the relationship between PLA and thermal traits was examined. The results showed a correlation between PLA, T50 (R2 = 0.466, P < 0.05), and T95 (R2 = 0.238, P < 0.1), suggesting that higher PLA is related to lower thermal values for T50 and T95, and therefore, decreased tolerance to heat stress. There has been no significant correlation between PLA and T5 (R2 = 0.026, P > 0.1). Yet, species with higher PLA tended to show lower thermal values (Fig. 9). No significant correlations were observed between the DWT95−T5, T50−T5, T95−T50 and PLA (Fig. 10).
Relationships between thermal tolerance traits and percentage loss of area (PLA). a The breaking point temperature (T5, broad-leaved trees (Traunstein): y = − 0.11x + 55.61, R2 = 0.026, P > 0.1, (b) the temperature of 50% decline of Fv/Fm (T50, broad-leaved trees (Traunstein): y = − 0.17 + 52.91, R2 = 0.43, P < 0.05, c the temperature of 95% decline of Fv/Fm (T95 broad-leaved trees (Traunstein): y = − 0.11 + 55.61, R2 = 0.24, P < 0.1). • means broad-leaved species (Traunstein), Δ conifers (Traunstein)
Relationships between percentage loss of area (PLA) and the decline width (DW). Three different temperature windows are present. (a) shows when Fv/Fm declines from 95 to 5% of the maximum Fv/Fm level (DWT95−T5, broad-leaved trees (Traunstein): y = − 0.29x + 25.45, R2 = 0.02, P > 0.1), b the Fv/Fm decline from 50 to 5% of the maximum Fv/Fm level (DWT50−T5, broad-leaved trees (Traunstein): y = -0.62x + 26.75, R2 = 0.07, P > 0.1), and c Fv/Fm declines from 95 to 50% of the maximum Fv/Fm m level (DWT95−T50, broad-leaved trees (Traunstein): y = 1.32x + 16.69, R2 = 0.08, P > 0.1). • means broad-leaved species (Traunstein), Δ conifers (Traunstein)
Discussion
The present study is the first comparative analysis of thermal and drought resistance traits in a wide range of mature temperate coniferous and broadleaved trees. The results demonstrate that thermal sensitivity is related to morphological and physiological traits that describe drought resistance. Species characterized by higher drought resistance tended to have higher breaking point temperatures than species characterized by lower drought resistance. This was further reflected in the thickness and toughness of the leaves, as species with higher xeromorphy were less sensitive to heat stress.
Dependence of thermotolerance on drought resistance traits
Our study suggests that the adaptation strategy of trees from temperate forests to extreme heat cannot be separated from their adaptation to resist drought stress. We found that species with higher thermal tolerance were characterized by physiological traits representing higher drought resistance, particularly πtlp (Kunert et al. 2021a; McGregor et al. 2021; Vargas et al. 2022). From an evolutionary perspective, a co-adaptation of temperate trees to both stressors, heat, and drought, is very likely for two reasons. First, periods of extreme heat and water limitation potentially co-occur during the summer (Kurjak et al. 2019), and second, extreme heat can induce water shortage due to increased atmospheric evaporative demand (Chaves et al. 2003; De Boeck and Verbeeck 2011). In this sense, Konôpková et al. (2018) suggested that a tree species' thermal sensitivity strongly depends on its drought avoidance strategy. Trees exposed to heat face the trade-off between cooling through transpiration, the risk to suffer from water limitation, or avoiding water stress by stomatal closure, but the risk of overheating foliage. Tree species characterized by a higher drought tolerance can be assumed to have evolved adaptations to resist higher temperatures. From a trait-based perspective, species with more negative πtlp can cool leaves during heat waves for a longer period than tree species with a less negative πtlp (Kunert et al. 2021a; Kunert and Hajek 2022) due to their ability of soil water uptake under more negative soil water potential. However, various studies suggest that a combination of drought and heat might affect the thermotolerance of trees. For example, pre-exposure to drought stress has been found to influence the thermostability of the PSII in trees (Epron 1997; Ladjal et al. 2000). An early summer drought might act as a trigger to enhance the thermostability of coniferous tree seedlings, and accordingly increase their thermotolerance (Ladjal et al. 2000). In turn, we suspect that with no pre-exposure to drought or in regions with high precipitation rates, forest trees might be especially susceptible to sudden heatwaves. Our field site in Traunstein is historically well supplied with sufficient rainfall due to the orographic lift in the vicinity of the alps. Evidence was found that the trees at the study site are characterized by a higher sensitivity to heat stress compared to trees at already drier sites. For example, Kunert and Hajek (2022) describe T5 and T50 values collected in the Vienna woods for the same species that are several degrees higher than at the Traunstein site (e.g., Sycamore maple: T5 = 41.7 °C in Vienna and 38.2 °C in this study). Indeed, both sites are characterized by climatic differences, as other factors than climate might also play a role, e.g., soil quality. Further, the combination of plant stress caused by heat and drought with other stressors, e.g., air pollution can have synergistic negative effects on plants and can produce somewhat similar responses in plants (Bormann 1985). The Traunstein plot received, on average, 1060 mm of rainfall per year, whereas the Vienna woods received less than 1000 mm and in the southern parts only 600 mm of rainfall. However, regional temperature differences might also be crucial in the intraspecific variation of thermal tolerance traits. Kurjak et al. (2019) found that European beech growing at warmer sites was characterized by a higher thermotolerance than at colder sites. This would also apply to the abovementioned example, as the mean annual temperature at Traunstein (7.3 °C) is much lower than at Vienna woods (10.9 °C).
Leaf functional traits and thermotolerance
This study presents the first evidence of a relationship between leaf functional traits and the leaf thermal sensitivity of tree species growing in a temperate forest with high precipitation rates. At our study site, thicker leaves (higher LMA) and leaves shrinking less upon desiccation (lower PLA) were characterized by a lower thermal sensitivity. Both higher LMA and PLA reflect a greater investment in leaf structural components resulting in more rigid leaves and leaf xeromorphy. Those attributes improve a species’ ability to withstand abiotic and biotic stress (Onoda et al. 2011). The observed relationship between functional leaf traits, particularly LMA, and thermal sensitivity has been described predominantly in hot and dry environments (Sastry et al. 2018). Despite the distinct different climatic conditions at our study site compared to the earlier study (Kunert and Hajek 2022), we found a significant relationship between LMA and thermal sensitivity, which is congruent with studies from a large variety of ecosystems worldwide (e.g., desert ecosystem, Curtis et al. 2014; tropical dry forest, Sastry and Barua 2017; desert and coastal ecosystem, Knight and Ackerly 2003). Most of the mentioned studies were conducted in, at least seasonally, arid and hot environments. Plants growing in arid and hot environments represent the highest end of xeromorphy, and xeromorphic leaves can be expected to have low thermal sensitivity.
In the literature, the variation of LMA is described to be linked to the “slow − fast” resource acquisition spectrum (Wright et al. 2004; Reich 2014). Further, we propose that LMA is a good indicator of the xeromorphic spectrum of an ecosystem, particularly with PLA as a co-explaining trait. In contrast to mesomorphic leaves, xeromorphic leaves show a reduced external area in relation to their thickness and are characterized by certain modifications in internal leaf structure (Shields 1950). For example, more xeromorphic leaves have a decreased cell size, thicker cell walls, and a denser network of veins. Furthermore, the palisade mesophyll has a great expansion relative to the spongy mesophyll (Schimper et al. 1903; Maximow and Krasnosselsky-Maximow 1924). These internal anatomical characteristics have important effects on the shrinking behavior of leaves upon desiccation, and xeromorphic leaves will shrink less upon desiccation than mesomorphic leaves. The shrinking behavior of leaves has been shown to affect the cell’s structural integrity and leaf hydraulic vulnerability (Scuffoni et al. 2014). PLA as a functional leaf trait represents a proxy of xeromorphy as it has been shown to predict growth reduction during drought in combination with πtlp (McGregor et al. 2021). Accordingly, a combination of LMA and PLA might be an easy and measurable approach to extend the current work on a larger species pool to choose species for the climate change adaption strategy in Central European Forests.
Diverging trends in trait relation in coniferous and broadleaved trees
Our study highlights the urgent need to implement large-scale climate-change adaptation to the managed forest in Central Europe. This study was conducted in an area that is currently not experiencing a significant reduction in precipitation. In the area, Norway spruce is currently the dominating tree species, and due to expected low shifts in precipitation, this species is not expected to be as heavily affected by drought-induced mortality as in other regions. However, Kunert (2020) proposed that the trigger for Norway spruce mortality is less related to drought-induced stress but more to low thermal tolerance. This hypothesis is supported by the finding that Norway spruce has a more negative πtlp of − 2.41 MPa than most species. A more negative πtlp indicates a higher drought tolerance (e.g., Bartlett et al. 2012; Kunert et al. 2021b; Vargas et al. 2022), and Norway spruce were accordingly even more leaf-level drought resistant than common oak (− 2.23 MPa) and almost as resistant as European beech (− 2.58 MPa). In contrast, the thermal tolerance of Norway spruce, expressed as T50 (46.7 ± 0.4 °C), was at the lower end of the found values compared to the average T50 of the entire species pool (48.7 ± 0.4 °C). Kunert et al. (2021a) show the same relationship, a more negative πtlp combined with a comparably low thermal tolerance, identifying Norway spruce as a very heat-sensitive species. It should be noted that Kunert et al. (2021a) found a negative relationship between leaf thermal tolerance and πtlp in conifers. Conifer species that are more drought tolerant were found to be less thermal tolerant, while drought-sensitive species were found to be more thermal tolerant.
In this study, only three conifer species were available, so it was not possible to verify the previous findings of Kunert et al. (2021a). In the Traunstein study, a positive relationship between thermal tolerance and drought resistance traits was found in broadleaved species. It is thought that the difference in the direction of the relationship may be due to the different anatomy of conifer and broadleaved tree species. For example, evergreen coniferous leaves have extended leaf longevity (Norway spruce four to seven years and Silver fir up to 11 years in the lowlands) compared to broadleaved winter-deciduous leaves. This longer leaf longevity means a much higher investment into leaf structure from the beginning and is notable in our study’s LMA of the conifers. The LMA of the evergreen conifer leaves was distinctively different from the broadleaved trees (10 times higher than the highest LMA of the broadleaved species). Therefore, it is suggested that evergreen conifers and broadleaved tree species should be treated separately in studies focusing on thermal traits due to their differing leaf anatomy. Interestingly the thermal traits and LMA of broadleaved-like deciduous conifer leaves, like the leaves of the bald cypress, align well with the relationship commonly found among broadleaved species.
Conclusions
This study demonstrates that the species pool of a wet temperate forest contains a range of species with notable differences in thermal tolerance and thermal sensitivity. The leaf thermal properties of the species are largely explained by leaf functional and morphological traits and significantly increase with higher drought tolerance. The observed breaking point temperatures at the lower end towards drought-sensitive species indicate that various species are already negatively affected by peak temperatures during the summer. As those peak temperatures will be higher and more frequent in the future, heat waves will be a significant challenge in Central European forests. It is shown that broadleaved species with a less negative turgor loss point and low leaf mass per area are less resistant to heat and more sensitive to drought. Therefore, broadleaved species with more negative turgor loss points and high leaf mass per area should focus on climate change adaptation strategies. We conclude that species with drought-adapted foliage can potentially deal better with heat stress by maintaining transpirational cooling during climatic stress.
References
Akaike H (1974) A new look at the statistical model identification. IEEE T Automat Contr 19(6):716–723
Alizadeh MR, Adamowski J, Nikoo MR, AghaKouchak A, Dennison P, Sadegh M (2020) A century of observations reveals increasing likelihood of continental-scale compound dry-hot extremes. Sci Adv 6(39):eaaz4571.
Bartlett MK, Scoffoni C, Ardy R, Zhang Y, Sun S, Cao K, Sack L (2012) Rapid determination of comparative drought tolerance traits: using an osmometer to predict turgor loss point. Methods Ecol Evol 3(5):880–888
Berry J, Bjorkman O (1980) Photosynthetic response and adaptation to temperature in higher plants. Annu Rev Plant Physiol 31(1):491–543
Binks O, Meir P, Rowland L, da Costa AC, Vasconcelos SS, de Oliveira AA, Ferreira L, Mencuccini M (2016) Limited acclimation in leaf anatomy to experimental drought in tropical rainforest trees. Tree Physiol 36(12):1550–1561
Bormann FH (1985) Air pollution and forests: an ecosystem perspective. Bioscience 35:434–441
Bose AK, Scherrer D, Camarero JJ, Ziche D, Babst F, Bigler C, Bolte A, Dorado-Liñán I, Etzold S, Fonti P, Forrester DI, Gavinet J, Gazol A, de Andrés EG, Karger DN, Lebourgeois F, Lévesque M, Martínez-Sancho E, Menzel A, Neuwirth B, Nicolas M, Sanders TGM, Scharnweber T, Schröder J, Zweifel R, Gessler A, Rigling A (2021) Climate sensitivity and drought seasonality determine post-drought growth recovery of Quercus petraea and Quercus robur in Europe. Sci Total Environ 784:147222
Buras A, Menzel A (2018) Projecting tree species composition changes of European forests for 2061−2090 under RCP 4.5 and RCP 8.5 scenarios. Front Plant Sci 9:1986.
Bussotti F (2008) Functional leaf traits, plant communities and acclimation processes in relation to oxidative stress in trees: a critical overview. Global Change Biol 14(11):2727–2739
Chaves MM, Maroco JP, Pereira JS (2003) Understanding plant responses to drought from genes to the whole plant. Funct Plant Biol 30(3):239–264
Cornelissen JHC, Lavorel S, Garnier E, Díaz S, Buchmann N, Gurvich DE, Reich PB, Ht S, Morgan HD, van der Heijden MGA, Pausas JG, Poorter H (2003) A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust J Bot 51(4):335
Curtis EM, Knight CA, Petrou K, Leigh A (2014) A comparative analysis of photosynthetic recovery from thermal stress: a desert plant case study. Oecologia 175(4):1051–1061
Davies SJ, Abiem I, Abu Salim K, Aguilar S, Allen D, Alonso A, Anderson-Teixeira K, Andrade A, Arellano G, Ashton PS, Baker PJ, Baker ME, Baltzer JL, Basset Y, Bissiengou P, Bohlman S, Bourg NA, Brockelman WY, Bunyavejchewin S, Burslem DFRP, Cao M, Cárdenas D, Chang LW, Chang-Yang CH, Chao KJ, Chao WC, Chapman H, Chen YY, Chisholm RA, Chu C, Chuyong G, Clay K, Comita LS, Condit R, Cordell S, Dattaraja HS, de Oliveira AA, den Ouden J, Detto M, Dick C, Du X, Duque Á, Ediriweera S, Ellis EC, Obiang NLE, Esufali S, Ewango CEN, Fernando ES, Filip J, Fischer GA, Foster R, Giambelluca T, Giardina C, Gilbert GS, Gonzalez-Akre E, Gunatilleke IAUN, Gunatilleke CVS, Hao Z, Hau BCH, He F, Ni H, Howe RW, Hubbell SP, Huth A, Inman-Narahari F, Itoh A, Janík D, Jansen PA, Jiang M, Johnson DJ, Jones FA, Kanzaki M, Kenfack D, Kiratiprayoon S, Král K, Krizel L, Lao S, Larson AJ, Li Y, Li X, Litton CM, Liu Y, Liu S, Lum SKY, Luskin MS, Lutz JA, Luu HT, Ma K, Makana JR, Malhi Y, Martin A, McCarthy C, McMahon SM, McShea WJ, Memiaghe H, Mi X, Mitre D, Mohamad M, Monks L, Muller-Landau HC, Musili PM, Myers JA, Nathalang A, Ngo KM, Norden N, Novotny V, O’Brien MJ, Orwig D, Ostertag R, Papathanassiou K, Parker GG, Pérez R, Perfecto I, Phillips RP, Pongpattananurak N, Pretzsch H, Ren H, Reynolds G, Rodriguez LJ, Russo SE, Sack L, Sang W, Shue J, Singh A, Song GZM, Sukumar R, Sun IF, Suresh HS, Swenson NG, Tan S, Thomas SC, Thomas D, Thompson J, Turner BL, Uowolo A, Uriarte M, Valencia R, Vandermeer J, Vicentini A, Visser M, Vrska T, Wang X, Wang X, Weiblen GD, Whitfeld TJS, Wolf A, Wright SJ, Xu H, Yao TL, Yap SL, Ye W, Yu M, Zhang M, Zhu D, Zhu L, Zimmerman JK, Zuleta D (2021) ForestGEO: Understanding forest diversity and dynamics through a global observatory network. Biol Conserv 253:108907
de Boeck HJ, Verbeeck H (2011) Drought-associated changes in climate and their relevance for ecosystem experiments and models. Biogeosciences 8(5):1121–1130
Epron D (1997) Effects of drought on photosynthesis and on the thermotolerance of photosystem II in seedlings of cedar (Cedrus atlantica and C. libani). J Exp Bot 48(10):1835–1841.
European Union, Copernicus Climate Change Service (2022) OBSERVER: A wrap-up of Europe’s summer 2022 heatwave | Copernicus. https://www.copernicus.eu/en/news/news/observer-wrap-europes-summer-2022-heatwave [accessed on 24.10.2022].
ForestGEO (2017) Traunstein. https://forestgeo.si.edu/sites/europe/traunstein [accessed on 24.10.2022].
Giammarchi F, Cherubini P, Pretzsch H, Tonon G (2017) The increase of atmospheric CO2 affects growth potential and intrinsic water-use efficiency of Norway spruce forests: insights from a multi-stable isotope analysis in tree rings of two Alpine chronosequences. Trees 31(2):503–515
Greenwood S, Ruiz-Benito P, Martínez-Vilalta J, Lloret F, Kitzberger T, Allen CD, Fensham R, Laughlin DC, Kattge J, Bönisch G, Kraft NJB, Jump AS (2017) Tree mortality across biomes is promoted by drought intensity, lower wood density and higher specific leaf area. Ecol Lett 20(4):539–553
Groom PK, Lamont BB, Leighton S, Leighton P, Burrows C (2004) Heat damage in sclerophylls is influenced by their leaf properties and plant environment. Écoscience 11(1):94–101
Hammond WM, Williams AP, Abatzoglou JT, Adams HD, Klein T, López R, Sáenz-Romero C, Hartmann H, Breshears DD, Allen CD (2022) Global field observations of tree die-off reveal hotter-drought fingerprint for Earth’s forests. Nat Commun 13(1):1761
Holdridge LR (1947) Determination of world plant formations from simple climatic data. Science 105(2727):367–368
Húdoková H, Petrik P, Petek-Petrik A, Konôpková A, Leštianska A, Střelcová K, Kmeť J, Kurjak D (2022) Heat-stress response of photosystem II in five ecologically important tree species of European temperate forests. Biologia 77(3):671–680
Kattge J, Díaz S, Lavorel S, Prentice IC, Leadley P, Bönisch G, Garnier E, Westoby M, Reich PB, Wright IJ, Cornelissen JHC, Violle C, Harrison SP, van Bodegom PM, Reichstein M, Enquist BJ, Soudzilovskaia NA, Ackerly DD, Anand M, Atkin O, Bahn M, Baker TR, Baldocchi D, Bekker R, Blanco CC, Blonder B, Bond WJ, Bradstock R, Bunker DE, Casanoves F, Cavender-Bares J, Chambers JQ, Chapin Iii FS, Chave J, Coomes D, Cornwell WK, Craine JM, Dobrin BH, Duarte L, Durka W, Elser J, Esser G, Estiarte M, Fagan WF, Fang J, Fernandez-Mendez F, Fidelis A, Finegan B, Flores O, Ford H, Frank D, Freschet GT, Fyllas NM, Gallagher RV, Green WA, Gutierrez AG, Hickler T, Higgins SI, Hodgson JG, Jalili A, Jansen S, Joly CA, Kerkhoff AJ, Kirkup D, Kitajima K, Kleyer M, Klotz S, Knops JMH, Kramer K, Kühn I, Kurokawa H, Laughlin D, Lee TD, Leishman M, Lens F, Lenz T, Lewis SL, Lloyd J, Llusia J, Louault F, Ma S, Mahecha MD, Manning P, Massad T, Medlyn BE, Messier J, Moles AT, Müller SC, Nadrowski K, Naeem S, Niinemets Ü, Nöllert S, Nüske A, Ogaya R, Oleksyn J, Onipchenko VG, Onoda Y, Ordonez J, Overbeck G, Ozinga WA, Patino S, Paula S, Pausas JG, Penuelas J, Phillips OL, Pillar V, Poorter H, Poorter L, Poschlod P, Prinzing A, Proulx R, Rammig A, Reinsch S, Reu B, Sack L, Salgado-Negret B, Sardans J, Shiodera S, Shipley B, Siefert A, Sosinski E, Soussana JF, Swaine E, Swenson N, Thompson K, Thornton P, Waldram M, Weiher E, White M, White S, Wright SJ, Yguel B, Zaehle S, Zanne AE, Wirth C (2011) TRY- a global database of plant traits. Global Change Biol 17(9):2905–2935
Kikuzawa K, Onoda Y, Wright I, Reich P (2013) Mechanisms underlying global temperature-related patterns in leaf longevity. Global Ecol Biogeogr 22(8):982–993
Kitudom N, Fauset S, Zhou Y, Fan Z, Li M, He M, Zhang S, Xu K, Lin H (2022) Thermal safety margins of plant leaves across biomes under a heatwave. Sci Total Environ 806(Pt 2):150416
Knight CA, Ackerly DD (2003) Evolution and plasticity of photosynthetic thermal tolerance, specific leaf area and leaf size: congeneric species from desert and coastal environments. New Phytol 160(2):337–347
Konôpková A, Kurjak D, Kmeť J, Klumpp R, Longauer R, Ditmarová Ľ, Gömöry D (2018) Differences in photochemistry and response to heat stress between silver fir (Abies alba Mill.) provenances. Trees 32(1):73–86.
Krause GH, Gallé A, Virgo A, García M, Bucic P, Jahns P, Winter K (2006) High-light stress does not impair biomass accumulation of sun-acclimated tropical tree seedlings (Calophyllum longifolium Willd. and Tectona grandis L.f.). Plant Biol 8:31–41
Krause GH, Winter K, Krause B, Jahns P, García M, Aranda J, Virgo A (2010) High-temperature tolerance of a tropical tree, Ficus insipida: methodological reassessment and climate change considerations. Funct Plant Biol 37(9):890
Kunert N, Hajek P (2022) Shade-tolerant temperate broad-leaved trees are more sensitive to thermal stress than light-demanding species during a moderate heatwave. Trees Forest People 9:100282
Kunert N, Hajek P, Hietz P, Morris H, Rosner S, Tholen D (2021a) Summer temperatures reach the thermal tolerance threshold of photosynthetic decline in temperate conifers. Plant Biol 24(7):1254–1261
Kunert N, Zailaa J, Herrmann V, Muller-Landau HC, Wright SJ, Pérez R, McMahon SM, Condit RC, Hubbell SP, Sack L, Davies SJ, Anderson-Teixeira KJ (2021b) Leaf turgor loss point shapes local and regional distributions of evergreen but not deciduous tropical trees. New Phytol 230(2):485–496
Kunert N (2020) Preliminary indications for diverging heat and drought sensitivities in Norway spruce and Scots pine in Central Europe. iForest 13(1):89–91.
Kurjak D, Konôpková A, Kmeť J, Macková M, Frýdl J, Živčák M, Palmroth S, Ditmarová Ľ, Gömöry D (2019) Variation in the performance and thermostability of photosystem II in European beech (Fagus sylvatica L.) provenances is influenced more by acclimation than by adaptation. Eur J For Res 138(1):79–92.
La Riva EG, Olmo M, Poorter H, Ubera JL, Villar R (2016) Leaf Mass per Area (LMA) and its relationship with leaf structure and anatomy in 34 Mediterranean woody species along a water availability gradient. PLoS ONE 11(2):e0148788
Ladjal M, Epron D, Ducrey M (2000) Effects of drought preconditioning on thermotolerance of photosystem II and susceptibility of photosynthesis to heat stress in cedar seedlings. Tree Physiol 20(18):1235–1241
Leuschner C (2020) Drought response of European beech (Fagus sylvatica L.)—A review. Perspect Plant Ecol Evol Syst 47:125576
Liao Z, Zhang L, Nobis MP, Wu X, Pan K, Wang K, Dakhil MA, Du M, Xiong Q, Pandey B, Tian X (2020) Climate change jointly with migration ability affect future range shifts of dominant fir species in Southwest China. Divers Distrib 26(3):352–367
Maximow NA, Krasnosselsky-Maximow TA (1924) Wilting of plants in its connection with drought resistance. J Ecol 12(1):95
McGregor IR, Helcoski R, Kunert N, Tepley AJ, Gonzalez-Akre EB, Herrmann V, Zailaa J, Stovall AEL, Bourg NA, McShea WJ, Pederson N, Sack L, Anderson-Teixeira KJ (2021) Tree height and leaf drought tolerance traits shape growth responses across droughts in a temperate broadleaf forest. New Phytol 231(2):601–616
Medina E, Garcia V, Cuevas E (1990) Sclerophylly and oligotrophic environments: relationships between leaf structure, mineral sclerophylly and oligotrophic environments: relationships between leaf structure, mineral nutrient content, and drought resistance in tropical rain forests of the upper Rio Negro Region. Biotropica 22:51–64
Nardini A (2022) Hard and tough: the coordination between leaf mechanical resistance and drought tolerance. Flora 288:152023
Niinemets U (2001) Global-scale climatic controls of leaf dry mass per area, density, and thickness in trees and shrubs. Ecology 82(2):453
Onoda Y, Westoby M, Adler PB, Choong AMF, Clissold FJ, Cornelissen JHC, Díaz S, Dominy NJ, Elgart A, Enrico L, Fine PVA, Howard JJ, Jalili A, Kitajima K, Kurokawa H, McArthur C, Lucas PW, Markesteijn L, Pérez-Harguindeguy N, Poorter L, Richards L, Santiago LS, Sosinski EE, van Bael SA, Warton DI, Wright IJ, Wright SJ, Yamashita N (2011) Global patterns of leaf mechanical properties. Ecol Lett 14(3):301–312
Oppenheimer HR (1960) Adaptation to drought: Xerophytism. In: Plant-water Relationships in Arid and Semi-arid Conditions. Paris: UNESCO.
Ordoñez JC, van Bodegom PM, Witte JPM, Wright IJ, Reich PB, Aerts R (2009) A global study of relationships between leaf traits, climate and soil measures of nutrient fertility. Global Ecol Biogeogr 18(2):137–149
O’sullivan OS, Heskel MA, Reich PB, Tjoelker MG, Weerasinghe LK, Penillard A, Zhu L, Egerton JJG, Bloomfield KJ, Creek D, Bahar NHA, Griffin KL, Hurry V, Meir P, Turnbull MH, Atkin OK (2017) Thermal limits of leaf metabolism across biomes. Global Change Biol 23(1):209–223
R Core Team (2022) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
Reich PB (2014) The world-wide ‘fast-slow’ plant economics spectrum: a traits manifesto. J Ecol 102(2):275–301
Reich PB, Walters MB, Ellsworth DS (1992) Leaf life-span in relation to leaf, plant, and stand characteristics among diverse ecosystems. Ecol Monogr 62(3):365–392
Reich PB, Wright IJ, Lusk CH (2007) Predicting leaf physiology from simple plant and climate attributes: a global GLOPNET analysis. Ecol Appl 17(7):1982–1988
Ritz C, Baty F, Streibig JC, Gerhard D (2015) Dose-Response Analysis Using R. PLOS ONE 10:e0146021. http://journals.plos.org/plosone/article?id=https://doi.org/10.1371/journal.pone.0146021.
Rousi E, Kornhuber K, Beobide-Arsuaga G, Luo F, Coumou D (2022) Accelerated western European heatwave trends linked to more-persistent double jets over Eurasia. Nat Commun 13(1):3851
Ruiz-Pérez G, Launiainen S, Vico G (2019) Role of plant traits in photosynthesis and thermal damage avoidance under warmer and drier climates in boreal forests. Forests 10(5):398
Salomón RL, Peters RL, Zweifel R, Sass-Klaassen UGW, Stegehuis AI, Smiljanic M, Poyatos R, Babst F, Cienciala E, Fonti P, Lerink BJW, Lindner M, Martinez-Vilalta J, Mencuccini M, Nabuurs GJ, van der Maaten E, von Arx G, Bär A, Akhmetzyanov L, Balanzategui D, Bellan M, Bendix J, Berveiller D, Blaženec M, Čada V, Carraro V, Cecchini S, Chan T, Conedera M, Delpierre N, Delzon S, Ditmarová Ľ, Dolezal J, Dufrêne E, Edvardsson J, Ehekircher S, Forner A, Frouz J, Ganthaler A, Gryc V, Güney A, Heinrich I, Hentschel R, Janda P, Ježík M, Kahle HP, Knüsel S, Krejza J, Kuberski Ł, Kučera J, Lebourgeois F, Mikoláš M, Matula R, Mayr S, Oberhuber W, Obojes N, Osborne B, Paljakka T, Plichta R, Rabbel I, Rathgeber CBK, Salmon Y, Saunders M, Scharnweber T, Sitková Z, Stangler DF, Stereńczak K, Stojanović M, Střelcová K, Světlík J, Svoboda M, Tobin B, Trotsiuk V, Urban J, Valladares F, Vavrčík H, Vejpustková M, Walthert L, Wilmking M, Zin E, Zou J, Steppe K (2022) The 2018 European heatwave led to stem dehydration but not to consistent growth reductions in forests. Nat Commun 13(1):28
Sastry A, Barua D (2017) Leaf thermotolerance in tropical trees from a seasonally dry climate varies along the slow-fast resource acquisition spectrum. Sci Rep 7(1):11246
Sastry A, Guha A, Barua D (2018) Leaf thermotolerance in dry tropical forest tree species: relationships with leaf traits and effects of drought. AoB Plants 10(1):plx070.
Schimper AFW, Fisher WR, Groom P, Balfour IB (1903) Plant-geography upon a physiological basis. Clarendon Press, Oxford
Scoffoni C, Vuong C, Diep S, Cochard H, Sack L (2014) Leaf shrinkage with dehydration: coordination with hydraulic vulnerability and drought tolerance. Plant Physiol 164(4):1772–1788
Senf C, Lakes T (2011) Assessing the vulnerability and risk to heat wave events in Germany and building a basis for further modelling. Wiechmann Verlag, Geospatial Crossroads at GI-Forum, Salzburg
Senf C, Pflugmacher D, Zhiqiang Y, Sebald J, Knorn J, Neumann M, Hostert P, Seidl R (2018) Canopy mortality has doubled in Europe’s temperate forests over the last three decades. Nat Commun 9(1):4978
Senf C, Buras A, Zang CS, Rammig A, Seidl R (2020) Excess forest mortality is consistently linked to drought across Europe. Nat Commun 11(1):6200
Senf C, Seidl R, Poulter B (2022) Post-disturbance canopy recovery and the resilience of Europe’s forests. Global Ecol Biogeogr 31(1):25–36
Shields LM (1950) Leaf xeromorphy as related to physiological and structural influences. Bot Rev 16(8):399–447
Tiwari R, Gloor E, da Cruz WJA, Schwantes MB, Marimon-Junior BH, Reis SM, de Souza IA, Krause HG, Slot M, Winter K, Ashley D, Béu RG, Borges CS, Da Cunha M, Fauset S, Ferreira LDS, Gonçalves MDA, Lopes TT, Marques EQ, Mendonça NG, Noleto PT, de Oliveira CHL, Oliveira MA, Pireda S, Dos Santos Prestes NCC, Santos DM, Santos EB, da Silva ELS, de Souza IA, de Souza LJ, Vitória AP, Foyer CH, Galbraith D (2021) Photosynthetic quantum efficiency in south-eastern Amazonian trees may be already affected by climate change. Plant Cell Environ 44(7):2428–2439
Vargas GG, Kunert N, Hammond WM, Berry ZC, Werden LK, Smith-Martin CM, Wolfe BT, Toro L, Mondragón-Botero A, Pinto-Ledezma JN, Schwartz NB, Uriarte M, Sack L, Anderson-Teixeira KJ, Powers JS (2022) Leaf habit affects the distribution of drought sensitivity but not water transport efficiency in the tropics. Ecol Lett 25(12):2637–2650
Wright I, Westoby M, Reich P (2002) Convergence towards higher leaf mass per area in dry and nutrient-poor habitats has different consequences for leaf life span. J Ecol 90(3):534–543
Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JHC, Diemer M, Flexas J, Garnier E, Groom PK, Gulias J, Hikosaka K, Lamont BB, Lee T, Lee W, Lusk C, Midgley JJ, Navas ML, Niinemets U, Oleksyn J, Osada N, Poorter H, Poot P, Prior L, Pyankov VI, Roumet C, Thomas SC, Tjoelker MG, Veneklaas EJ, Villar R (2004) The worldwide leaf economics spectrum. Nature 428(6985):821–827
Acknowledgements
We thank Tobias Steiner for the hospitality and permission to set up our field laboratory in the Froschham hermitage. We thank Gabrielle Arellano for providing the R-script for the leaf area analysis. We are in debt to two anonymous reviewers for their valuable comments on how to improve the manuscript. We are grateful to Dr. Alida Mercado Cardenas for language editing of the final manuscript.
Funding
Open access funding provided by University of Natural Resources and Life Sciences Vienna (BOKU).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Corresponding editor: Yanbo Hu.
Guest editor: Yanbo Hu.
Project funding: This study was funded by a DIBB research stimulation grant.
The online version is available at http://www.springerlink.com.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Münchinger, I., Hajek, P., Akdogan, B. et al. Leaf thermal tolerance and sensitivity of temperate tree species are correlated with leaf physiological and functional drought resistance traits. J. For. Res. 34, 63–76 (2023). https://doi.org/10.1007/s11676-022-01594-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-022-01594-y