Abstract
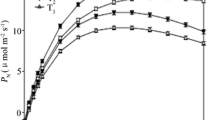
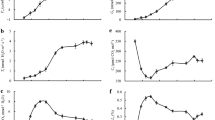
We applied under pot-culture conditions and the double-casing pot method to study the characteristics of photosynthetic gas exchange and chlorophyll fluorescence in the leaves of Physocarpus amurensis Maxim (PA) and Physocarpus opulifolius under flooding stress. Our results indicate a significantly higher flooding tolerance of P. opulifolius compared to P. amurensis. Especially in P. amurensis, the limitation of non-stomatal factors played a major role in the advanced stages of flooding stress, observed as a rapid increase of the intercellular CO2 concentration (C i) and a decrease of the stomatal limitation value (L s). The maximal PSII photochemical efficiencies (F v/F m) and actual photochemical efficiency (Ф PSII) in the leaves of P. opulifolius were significantly higher, and the extent of decrease during the flooding process was smaller than in P. amurensis. In addition, the non-chemical quenching (NPQ) in the leaves of P. opulifolius significantly increased from the 10th day under flooding stress, while the variation of NPQ in the leaves of P. amurensis was much smaller. This indicates that the leaves of P. opulifolius had not only higher PSII photochemical activity, but also improved tolerance to flooding stress, which may be caused by its ability to dissipate excess excitation energy by starting NPQ. At the 16th day under flooding stress, the P IABS significantly decreased with greater extent of decrease than F v/F m in the leaves of both Physocarpus, but the decreasing extent of P IABS in P. opulifolius was significantly smaller than in P. amurensis. In the 16th day under flooding stress, the fluorescence at J and I point (V J and V I) in P. amurensis were significantly higher, and the extent of increase in V J was greater than V I. However, the variations of V J and V I in the leaves of P. opulifolius were smaller, suggesting that the damage sites of flooding stress to PSII in the leaves of P. amurensis were mainly located in the electron transport process from QA at the PSII receptor side to QB. Flooding stress reduced the proportion (φE o ) of luminous energy absorbed by the PSII reaction center for the electron transport following Q −A , while the maximum quantum yield (φD o) of non-photochemical quenching increased. However, the TRo/RC and ETo/RC in the leaves of P. amurensis decreased accompanied by a dramatic increase of energy (DIo/RC) from the dissipation in the reaction center. This further indicated that the function of the PSII reaction center in the leaves of P. amurensis was significantly lower than in P. opulifolius.







Similar content being viewed by others
References
Abiko T, Kotuia L, Shiono K, Malik A, Colmer TD, Nakazono M (2012) Enhanced formation of aerenchyma and induction of a barrier to radial oxygen loss in adventitious roots of Zea nicaraguensis contribute to its waterlogging tolerance as compared with maize. Plant, Cell Environ 35(9):1618–1630
Ahmed S, Nawata E, Hosokawa M, Domae Y, Sakuratani T (2002) Alterations in photosynthesis and some antioxidant enzymatic activities of mungbean subjected to waterlogging. Plant Sci 163(1):117–123
Arbona V, Hossain Z, Clemente RP, Cadenas AG (2008) Antioxidant enzymatic activity is linked to waterlogging stress tolerance in citrus. Physiol Plant 132(4):452–466
Ashraf M, Arfan M (2005) Gas exchange characteristics and water relations in two cultivars of Hibiscus esculentus under Waterlogging. Biol Plant 49(3):459–462
Cao G, Wang XG, Liu Y, Luo W (2012) Effect of water logging stress on cotton leaf area index and yield. Proc Eng 28(12):202–209
Chartzoulakis K, Klapaki G (2000) Response of two greenhouse pepper hybrids to NaCl salinity during different growth stages. Sci Horti 86(3):247–260
Chaves MM, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot 103(4):551–560
Cheng DD, Liu MJ, Sun XB, Zhao M, Chow WS, Sun GY (2016a) Light suppresses bacterial population through the accumulation of hydrogen peroxide in tobacco leaves infected with pseudomonas syringaepv.tabaci. Front. Plant Sci 7(512):1–11
Cheng DD, Zhang ZS, Sun XB, Zhao M, Sun GY (2016b) Photoinhibition and photoinhibition-like damage to the photosynthetic apparatus in tobacco leaves induced bypseudomonas syringaepv.Tabaciunder light and dark conditions. BMC Plant Biol 16(1):1–11
Du KB, Xu L, Tu BK, Shen BX (2010) Influences of soil flooding on ultrastructure and photosynthetic capacity of leaves of one-year old seedlings of two poplar clones. Sci Silv Sin 46(6):58–64
Fan XL, Zhang ZS, Gao HY, Yang C, Liu MJ, Li PM (2014) Photoinhibition-like damage to the photosynthetic apparatus in plant leaves induced by submergence treatment in the dark. PLoS ONE 9(2):1633–1641
Farquhar GD, Sharkey TD (1982) Stomatal conductance and photosynthesis. Ann Rev Plant Physiol 33:317–345
Fernandez MD (2006) Changes in photosynthesis and fluorescence in response to flooding in emerged and submerged leaves of Pouteria orinocoensis. Photosynthetica 44(1):32–38
Fukao T, Bailey-Serres J (2004) Plant responses to hypoxia: issurvival a balancing act. Trends Plant Sci 9(9):449–456
Fukao T, Bailey-Serres J (2008) Ethylene-A key regulator of submergence responses in rice. Plant Sci 175(S1–2):43–51
Geigenberger P (2003) Response of plant metabolism to too little oxygen. Cur Opin Plant Biol 6(3):247–256
Hank W, William A, Timothyd C (2006) Conditions leading to high CO2 (> 5 kPa) in waterlogged flooded soils and possible effects on root growth and metabolism. Ann Bot 98(1):9–32
Havaux M, Davaud A (1994) Photoinhibition of photosynthesis in chilled potato leaves is not correlated with a loss of photosystem-II activity-preferential inactivation of photosystem I. Photosynth Res 40:75–92
Hu YB, Sun GY, Wang XC (2007) Induction characteristics and response of photosynthetic quantum conversion to changes in irradiance in mulberry plants. J Plant Physiol 164(8):959–968
Huang SB, Colmer TD, Millar AH (2008) Does anoxia tolerance involve altering the energy currency towards PPi? Trends Plant Sci 13(5):221–227
Islam MA, Macdonald SE (2004) Ecophysiological adaptations of black spruce (Picea mariana) and tamarack (Larix laricina) seedlings to flooding. Trees 18(18):35–42
Jackson MB, Armstrong W (1999) Formation of aerenchyma and the processes of plant ventilation in relation to soil flooding and submergence. Plant Biol 1(3):274–287
Kaelke CM, Dawson JO (2003) Seasonal flooding regimes influence survival, nitrogen fixation and the partitioning of nitrogen and biomass in Alnus incana ssp. Rugosa. J Plant Soil 254(1):167–177
Kreslavski VD, Carpentier R, Klimov VV, Murata N, Allakhverdiev SI (2007) Molecular mechanisms of stress resistance of the photosynthetic apparatus. Biochem Suppl Ser A Membr Cell Biol 1(3):185–205
Li PM, Cheng LL, Gao HY, Peng T (2000a) Heterogeneous behavior of PSII in soybean (Glycine max) leaves with identical PSII photochemistry effi ciency under different high temperature treatments. J Plant Physiol 166(15):1607–1615
Li XP, Bjorkman O, Shi C, Grossman AR, Rosenquist M, Jansson S, Niyogi KK (2000b) A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature 403(6768):391–395
Li HB, Chen WF, Li QY (2006) Responses of rice leaf photosynthetic parameters to light intensity under NaCl stress. Chin J Appl Ecol 17(9):1588–1592
Li G, Gao HY, Zhao B, Dong ST, Zhang JW, Yang JS, Wang JF, Liu P (2009a) Effects of drought stress on activity of photosystems in leaves of maize at grain filling stage. Acta Agron Sin 35(10):1916–1922
Li PM, Cheng LL, Gao HY, Jiang C, Peng T (2009b) Heterogeneous behavior of PSII in soybean (Glycine max) leaves with identical PSII photochemistry efficiency under different high temperature treatments. J Plant Physiol 166(15):1607–1615
Li Y, Du YP, Fu YD, Heng D (2013) Physiological responses of waterlogging on different rootstock combinations of cabernet sauvignon grape. Acta Hortic Sin 40(11):2105–2114
Lindroth RL, Reich PB, Tjoelker MG, Volin JC, Oleksyn J (1993) Light environment alters response to ozone stress in seedlings of Acer saccharum Marsh, and hybrid Populus L. New Phytol 124(4):637–646
Liu MJ, Zhang ZS, Gao HY, Cheng Y, Fan XL, Cheng DD (2014) Effect of leaf dehydration duration and dehydration degree on psII photochemical activity of papaya leaves. Plant Physiol Biochem 82(3):85–88
Lu CM, Qiu NW, Wang BS, Zhang J (2003) Salinity treatment shows no effects on photosystem II photochemistry, but increases the resistance of photosystem II to heat stress in halophyte Suaeda salsa. J Exp Bot 54(383):851–860
Mathur S, Agrawal D, Jajoo A (2014) Photosynthesis: response to high temperature stress. J Photochem Photobiol Biol 137(8):116–126
Meyer S, Kouchkovsky Y (1993) Electron transport, photosystem-reaction centers and chlorophyll protein complexes of thylakoids of drought resistant and sensitive lupin plants. Photosynth Res 37(1):49–60
Nadia AA, Dewez D, Didur O, Popovic R (2006) Inhibition of photosystem II photochemistry by Cr is caused by the alteration of both D1 protein and oxygen evolving complex. Photosynth Res 89(89):81–87
Nishiuchi S, Yamauchi T, Takahashi H, Kotual L, Nakazono M (2012) Mechanisms for coping with submergence and waterlogging in rice. Rice 5(2):1–14
Olgun M, Kumlay AM, Adiguzel MC, Abdullah C (2008) The effect of waterlogging in wheat (Triticum aestivum L.). Acta Agric Scand Sect B Soil Plant Sci 58(3):193–198
Parolin P, Armbrüster N, Junk WJ (2006) Two Amazonian floodplain trees react differently to periodical flooding. Trop Ecol 47(2):243–250
Prasad TK, Anderson MD, Martin BA, Stewart CR (1994) Evidence for chilling-induced oxidative stress in maize seedlings and a regulatory role for hydrogen peroxide. Plant Cell 6(1):65–74
Qi L, Ma N, Wu WW, An YY, Xu JC, Qin XH, Wang LJ (2015) Physiological responses and tolerance evaluation of fig cultivars to waterlogging. Acta Hortic Sin 42(7):1273–1284
Qu NW, Zhou F, Gu ZJ, Jia SQ, Wang XA (2012) Photosynthetic functions and chlorophyll fast fluorescence characteristics of five Pinus species. Chin J Appl Ecol 23(5):1181–1187
Salmela MJ, Cavers S, Cottrell JE, Lason GR, Ennos RA (2011) Seasonal patterns of photochemical capacity and spring phenology reveal genetic differentiation among native Scots pine (Pinus sylvestris L.) populations in Scotland. For Ecol Manag 262(6):1020–1029
Satoh K, Smith CM, Fork DC (1983) Effect of salinity on primary processes of photosynthesis in the red alga porphyraperforata. Plant Physiol 73(3):643–647
Seemann JR, Sharkey TD (1986) Salinity and nitrogen effects on photosynthesis, ribulose-1,5-bisphosphate carboxylase and metabolite pool sizes in Phaseolus vulgaris L. Plant Physiol 82(2):555–560
Strasser RJ, Srivastava A, Govindjee (1997) Polyphasic chlorophyll a fluorescence transients. Photosynth Res 52(2):147–155
Strauss AJ, Kruger GHJ, Strasser RJ, Heerden PDRV (2006) Ranking of dark chilling tolerance in soybean genotypes probed by the chlorophyll a fluorescence transient O–J–I–P. Environ Exp Bot 56(2):147–157
Ushimaru T, Ogawa K, Ishida N, Tsuji H (1995) Changes in organelle superoxide dismutase isoenzymes during air adaptation of submerged rice seedlings: Differential behavior of isoenzymes in plastids and mitochondoria. Planta 196(3):606–613
Yan K, Chen P, Shao H, Zhao S, Zhang L, Zhang L, Xu G, Sun J (2012) Responses of photosynthesis and photosystem IIto higher temperature and salt stress in sorghum. J Agron Crop Sci 198(3):218–226
Yavas I, Unaya A, Aydin M (2012) The waterlogging tolerance of wheat varieties in western of Turkey. Sci World J 4:1–7
Yu YY, Zhang HY, Pan J, Tan Z, Ma L (2010) Cross-breedingof Physocarpus plant. J Northeast For Uni 38(7):16–18
Yuan L, Zhang LQ, Gu ZQ (2012) Responses of chlorophyll fluoresescence of aninsive plant Spartina alterniflorato continuous waterlogging. Acta Sci Circ 30(4):882–889
Zhang XK, Zhou QH, Cao JH, Yu BJ (2011) Differential Cl−/Salt tolerance and NaCl-induced alternations of tissue and cellular ion fluxes in Glycine max, Glycine soja and their Hybrid Seedlings. J Agron Crop Sci 197(5):1–11
Zhang HH, Zhang XL, Li X, Ding JN, Zhu WX, Qi F, Zhang T, Tian Y, Sun GY (2012a) Effects of NaCl and Na2CO3 stresses on the growth and photosynthesis characteristics of Morus alba seedlings. Chin J Appl Ecol 23(3):625–631
Zhang ZS, Li G, Gao HY, Zhang LT, Yang C, Liu P, Meng QW (2012b) Characterization of photosynthetic performance during senescence in stay-green and quick-leaf-senescence Zea mays L. Inbred Lines. PLoS ONE 7(8):e42936
Zhang BB, Xu JL, Cai ZX, Ma RJ (2013) Relationship between photosynthetic characteristics and environmental factors in leaves of two plum rootstock varieties under waterlogging stress. J Nanjing Agric Univ 36(5):39–44
Zhang HH, Zhong HX, Sui X, Xu N (2016) Adaptive changes in chlorophyll content and photosynthetic features to low light in physocarpus amurensis maxim and physocarpus opulifolius “diabolo”. Peer J 4(3):e2125
Zhou YZ (2000) Studies on the propagation in vitro of Physocorpus opulifolius “Lutein”. Acta Hortic Sin 27(2):148–150
Author information
Authors and Affiliations
Corresponding authors
Additional information
Project funding: This study was supported by the National Natural Science Foundation of China (No. 31500323).
The online version is available at http://www.springerlink.com
Corresponding editor: Zhu Hong.
Rights and permissions
About this article
Cite this article
Zhang, H., Feng, P., Yang, W. et al. Effects of flooding stress on the photosynthetic apparatus of leaves of two Physocarpus cultivars. J. For. Res. 29, 1049–1059 (2018). https://doi.org/10.1007/s11676-017-0496-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-017-0496-2