Abstract
Objective
To evaluate the efficacy and safety of Tongxiening Granules (痛泻宁颗粒, TXNG) in the treatment of irritable bowel syndrome with predominant diarrhea (IBS-D).
Methods
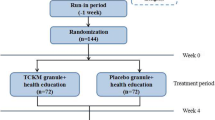
A randomized, double-blind, double-dummy, and positive parallel controlled clinical trial was conducted from October 2014 to March 2016. Totally 342 patients from 13 clinical centers were enrolled and randomly assigned (at the ratio of 1:1) to a treatment group (171 cases) and a control group (171 cases) by a random coding table. The patients in the treatment group were administered orally with TXNG (5 g per time) combined with pinaverium bromide Tablet simulator (50 mg per time), 3 times per day. The patients in the control group were given TXNG simulator (5 g per time) combined with pinaverium bromide Tablets (50 mg per time), 3 times per day. The treatment course lasted for 6 weeks. The improvement of Irritable Bowel Syndrome Symptom Severity Score (IBS-SSS) was used to evaluate the primary outcome. Secondary outcomes included adequate relief (AR) rate, Irritable Bowel Syndrome-Quality of Life Questionnaire (IBS-QOL), Hamilton Anxiety Scale (HAMA), Hamilton Depression Scale (HAMD), and the recurrence rate at follow-ups. Safety indices including the adverse events (AEs) and related laboratory tests were evaluated.
Results
Primary outcome: IBS-SSS at baseline, weeks 2, 4, 6 showed no statistical significance in both full analysis set (FAS) and per protocol set (PPS, P>0.05). After 6 weeks of treatment, the total effective rate of IBS-SSS scores in the treatment group (147/171,86.0%) was higher than the control group (143/171, 83.6%) by FAS (P>0.05). In regard to secondary outcomes, after 6-week treatment, there was no significant difference in AR rate, total score of IBS-QOL, improvement of HAMD and HAMA total scores between the two groups (P>0.05). The recurrence rate at 8-week follow-up was 12.35% (10/18) in treatment group and 15.79% (12/76) in control group, respectively (P>0.05). A total of 21 AEs occurred in 15 cases, of which 11 occurred in 8 cases in the treatment group and 10 AEs in 7 cases in the control group. The incidence of AEs had no statistical significance between the two goups (P>0.05).
Conclusion
Tongxiening Granules could relieve the symptoms of patients with IBS-D and the treatment effect was comparable to pinaverium bromide. (No. ChiCTR-IPR-15006415)
Similar content being viewed by others
References
Xiong LS, Chen MH, Chen HX, Xu AG, Wang WA, Hu PJ. A population-based epidemiologic study of irritable bowel syndrome in Guangdong Province. Natl Med J China (Chin) 2004;84:278–281.
Wang G, Li TQ, Wang L, Xia Q, Chen Y, Zhang RM. A randomized, double-blind, placebo control trial of Tongxiening Granules for the treatment of diarrhea-predominant irritable bowel syndrome (stagnation of the Liver-qi attacking the Spleen). Chin J Evid-Based Med (Chin) 2006;6:84–89.
Tang XD, Lu B, Li ZH, Wei W, Meng LN, Li BS, et al. Therapeutic effect of Chang’an I Recipe on irritable bowel syndrome with diarrhea: a multicenter randomized double-blind placebo-controlled clinical trial. Chin J Integr Med 2018;24:645–652.
Tongxiening Granules Research and Cooperation Group. A randomized, double-blind, placebo control, multicenter clinical trial of Tongxiening Granules for the treatment of diarrhea-predominant irritable bowel syndrome. Chin J Dig (Chin) 2010;30:327–330.
Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology 2006;130:1480–1491.
Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther 1997;11:395–402.
Bian LQ, Lu F, Li ZH, Li BS, Gao R, Wang FW, et al. Analysis of response of IBS-SSS, AR, and IBS-QOL in IBS clinical effect evaluation. Chin J Integr Tradit West Med (Chin) 2016;36:1191–1196.
Camilleri M, Mayer EA, Drossmana A. Improvement in pain and bowel function in female irritable bowel patients with alosetron, a 5-HT3 receptor antagonist. Aliment Pharmacol Ther 1999;13:1149–1159.
Huang WW, Zhou FS, Bushnell DM, Diakite C, Yang XH. Cultural adaptation and application of the IBS-QOL in China: a disease-specific quality-of-life questionnaire. Qual Life Res 2007;16:991–996.
Hamilton. The assessment of anxiety states by rating. Br J Med Psychol 1959;3:50–55.
Hamilton. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56–62.
Dai N. Research progress of the relationship between 5-hydrocytryptamine and irritable bowel syndrome. Intern J Dig Dis (Chin) 2010;30:1–3.
Li DG, Zhao YB. Experimental study on the functional mechanism of pain and diarrhea formula on irritable bowel syndrome. Chin Herb Med (Chin) 2006;37:1681–1685.
Liu ZJ, Zhao YB. Study on the pain and diarrhea formula in inhibiting the abdominal mast cells degranulation of the sensitized rats. Modern J Integr Tradit Chin West Med (Chin) 2007;16:1900–1903.
Zhou ZH, Ji JB, Wang ZC. Effects of Shaoyao Gancao Decoction on visceral hypersensitivity of irritable bowel syndrome rats. Chin J Integr Tradit West Med (Chin) 2017;37:575–578.
Tian SY, Zheng GQ, Wei SC, Song H, Li CY. Effects of Tongxiening Granules on peripheral serum IL-18/ IL-10 of patients with IBS-D. Chongqing Med (Chin) 2013;42:1084–1088.
Yin PF, He YF. Efficacy of Tongxiening Granules on diarrhea-predominant irritable bowel syndrome and its effect on peripheral serum IL-18 and IL-10. Modern J Integr Tradit Chin West Med (Chin) 2014;23:2447–2448.
Yang YL, Zheng TZ, Zhai SY, Li W, Xie DP, Ding YH; et al. The effects of Pericarpium citri reticulatae viride and Pericarpium citri reticulatae on the small intestinal longitudinal muscle strips movement of rats. J Lanzhou Univ: Nat Sci Edit (Chin) 2001;37:94–97.
Chen XX. Preliminary study on the antibacterial activity of allium macrostemon. J Hangzhou Normal Univ (Nat Sci Edit, Chin) 2004;3:337–340.
Author information
Authors and Affiliations
Contributions
Tang XD and Li ZH designed the study; Zhang SS, Hou XH, Chen S, Feng PM, Yang XN, Li HZ, Wu JQ, Xia PJ, Yang XJ, Zhou HJ, Wang HY and Ai YW carried out the trial; Li K performed the statistical analysis; Tang XD designed and wrote the manuscript.
Corresponding author
Additional information
Conflict of Interest
The authors declare no conflict of interest with respect to the research, authorship, and/or publication of this article. This study was funded by Chongqing Pharscin Pharmaceutical Co., Ltd.
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Tang, Xd., Zhang, Ss., Hou, Xh. et al. Post-marketing Re-evaluation of Tongxiening Granules (痛泻宁颗粒) in Treatment of Diarrhea-Predominant Irritable Bowel Syndrome: A Multi-center, Randomized, Double-Blind, Double-Dummy and Positive Control Trial. Chin. J. Integr. Med. 25, 887–894 (2019). https://doi.org/10.1007/s11655-019-3030-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11655-019-3030-x