Abstract
Objective
To evaluate the potential pharmacokinetic interactions of the anticancer agent gefitinib (Iressa®) and the oriental medications Guipi Decoction (归脾汤, GPD, Guibi-tang in Korean) and Bawu Decoction (八物汤, BWD, Palmul-tang in Korean).
Methods
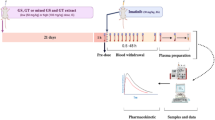
Methylcellulose (MC, control), GPD (1,200 mg/kg), or BWD (6,000 mg/kg) was orally administered to rats either as a single dose or multiple doses prior to gefitinib administration. To examine the effects of a single dose of the herbal medicines, gefitinib (10 mg/kg) was orally administered after 5 min or 1 h of MC or the herbal medicine pretreatments. To examine the effects of the multiple doses of the herbal medicines, gefitinib (10 mg/kg) was orally administered following 7 consecutive days of the administration of MC or each herbal medicine. The plasma concentrations of gefitinib were determined with liquid chromatography-tandem mass spectrometry assay. The plasma concentration-time profiles of gefitinib were analyzed with a noncompartmental analysis.
Results
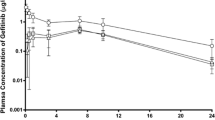
Gefitinib was rapidly absorbed and showed a monoexponential decline with an elimination half-life of 3.7–4.1 h. The pharmacokinetics of gefitinib was not affected by GPD pretreatment. However, a significantly lower maximum plasma concentration (Cmax, P<0.05) and area under the curve (P<0.05), and a delayed time to reach Cmax (Tmax, P<0.01) were observed in both single- and multipledose BWD-pretreated rats compared with the control rats.
Conclusions
BWD and not GPD might delay and interfere with gefitinib absorption. Further evaluations of the clinical significance of these findings are needed.
Similar content being viewed by others
References
Chan K. Understanding interactions between Chinese medicines and pharmaceutical drugs in integrative healthcare. Chin J Integr Med 2015;21:83–89.
Xu H, Chen KJ. Herb-drug interaction: an emerging issue of integrative medicine. Chin J Integr Med 2010;16:195–196.
Boon HS, Olatunde F, Zick SM. Trends in complementary/alternative medicine use by breast cancer survivors: comparing survey data from 1998 and 2005. BMC Womens Health 2007;7:4.
Yang AK, He SM, Liu L, Liu JP, Wei MQ, Zhou SF. Herbal interactions with anticancer drugs: mechanistic and clinical considerations. Curr Med Chem 2010;17:1635–1678.
Werneke U, Earl J, Seydel C, Horn O, Crichton P, Fannon D. Potential health risks of complementary alternative medicines in cancer patients. Br J Cancer 2004;90:408–413.
Cohen MH, Williams GA, Sridhara R, Chen G, McGuinn WD Jr., Morse D, et al. United States Food and Drug Administration Drug Approval summary: Gefitinib (ZD1839; Iressa) tablets. Clin Cancer Res 2004;10:1212–1218.
Joshi M, Rizvi SM, Belani CP. Afatinib for the treatment of metastatic non-small cell lung cancer. Cancer Manag Res 2015;7:75–82.
McKillop D, McCormick AD, Millar A, Miles GS, Phillips PJ, Hutchison M. Cytochrome P450-dependent metabolism of gefitinib. Xenobiotica 2005;35:39–50.
Swaisland HC, Ranson M, Smith RP, Leadbetter J, Laight A, McKillop D, et al. Pharmacokinetic drug interactions of gefitinib with rifampicin, itraconazole and metoprolol. Clin Pharmacokinet 2005;44:1067–1081.
Galetti M, Alfieri RR, Cavazzoni A, La Monica S, Bonelli M, Fumarola C, et al. Functional characterization of gefitinib uptake in non-small cell lung cancer cell lines. Biochem Pharmacol 2010;80:179–187.
Zimmerman EI, Hu S, Roberts JL, Gibson AA, Orwick SJ, Li L, et al. Contribution of OATP1B1 and OATP1B3 to the disposition of sorafenib and sorafenib-glucuronide. Clin Cancer Res 2013;19:1458–1466.
Agarwal S, Sane R, Gallardo JL, Ohlfest JR, Elmquist WF. Distribution of gefitinib to the brain is limited by P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2)-mediated active efflux. J Pharmacol Exp Ther 2010;334:147–155.
Elkind NB, Szentpetery Z, Apati A, Ozvegy-Laczka C, Varady G, Ujhelly O, et al. Multidrug transporter ABCG2 prevents tumor cell death induced by the epidermal growth factor receptor inhibitor Iressa (ZD1839, Gefitinib). Cancer Res 2005;65:1770–1777.
Ozvegy-Laczka C, Cserepes J, Elkind NB, Sarkadi B. Tyrosine kinase inhibitor resistance in cancer: role of ABC multidrug transporters. Drug Resist Updat 2005;8:15–26.
Nakamura Y, Oka M, Soda H, Shiozawa K, Yoshikawa M, Itoh A, et al. Gefitinib ("Iressa", ZD1839), an epidermal growth factor receptor tyrosine kinase inhibitor, reverses breast cancer resistance protein/ABCG2-mediated drug resistance. Cancer Res 2005;65:1541–1546.
Leggas M, Panetta JC, Zhuang Y, Schuetz JD, Johnston B, Bai F, et al. Gefitinib modulates the function of multiple ATP-binding cassette transporters in vivo. Cancer Res 2006;66:4802–4807.
Shin HY, An NH, Cha YJ, Shin EJ, Shin TY, Baek SH, et al. Effect of Kuibitang on lipopolysaccharide-induced cytokine production in peripheral blood mononuclear cells of chronic fatigue syndrome patients. J Ethnopharmacol 2004;90:253–259.
Ha JY, Nam WY. Experimental studies on antitumor and immunomodulatory effects of Palmultang. J Orient Med Pathol 1995;9:295–315.
Ha J, Baik TH. Effect of Palmul-tang on lactate tolerance and recovery rate in the human body. J Orient Chr Dis 2000;6:184–196.
Heo M, Hong H, Kam C, Park D. Experimental study on the anti-allergic effects of Palmul-tang. Kor J Ori Med Physiol Pathol 2003;17:1075–1081.
Yim NH, Kim A, Liang C, Cho WK, Ma JY. Guibitang, a traditional herbal medicine, induces apoptotic death in A431 cells by regulating the activities of mitogen-activated protein kinases. BMC Complement Altern Med 2014;14:344.
Lee B, Weon JB, Yun BR, Lee J, Eom MR, Ma CJ. Simultaneous determination of 11 major components in Palmul-tang by HPLC-DAD and LC-MS-MS. J Chromatogr Sci 2014;52:482–492.
McKillop D, Partridge EA, Hutchison M, Rhead SA, Parry AC, Bardsley J, et al. Pharmacokinetics of gefitinib, an epidermal growth factor receptor tyrosine kinase inhibitor, in rat and dog. Xenobiotica 2004;34:901–915.
McKillop D, Hutchison M, Partridge EA, Bushby N, Cooper CM, Clarkson-Jones JA, et al. Metabolic disposition of gefitinib, an epidermal growth factor receptor tyrosine kinase inhibitor, in rat, dog and man. Xenobiotica 2004;34:917–934.
Bergman E, Forsell P, Persson EM, Knutson L, Dickinson P, Smith R, et al. Pharmacokinetics of gefitinib in humans: the influence of gastrointestinal factors. Int J Pharm 2007;341:134–142.
Mandery K, Balk B, Bujok K, Schmidt I, Fromm MF, Glaeser H. Inhibition of hepatic uptake transporters by flavonoids. Eur J Pharm Sci 2012;46:79–85.
Mandery K, Bujok K, Schmidt I, Keiser M, Siegmund W, Balk B, et al. Influence of the flavonoids apigenin, kaempferol, and quercetin on the function of organic anion transporting polypeptides 1A2 and 2B1. Biochem Pharmacol 2010;80:1746–1753.
Zhang S, Yang X, Morris ME. Flavonoids are inhibitors of breast cancer resistance protein (ABCG2)-mediated transport. Mol Pharmacol 2004;65:1208–1216.
Taur JS, Rodriguez-Proteau R. Effects of dietary flavonoids on the transport of cimetidine via P-glycoprotein and cationic transporters in Caco-2 and LLC-PK1 cell models. Xenobiotica 2008;38:1536–1550.
Rathee P, Rathee S, Ahuja D. Simultaneous quantification of glycyrrhetinic acid & apigenin using HPTLC from Glycyrrhiza glabra Linn. Eurasian J Analyt Chem 2010;5:95–103.
Jun YM, Kim EH, Lim JJ, Kim SH, Kim SH, Lim JD, et al. Variation of phenolic compounds contents in cultivated Astragalus membranaceus. Kor J Med Crop Sci 2012;20:447–453.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Weon, KY., Kim, M.G., Shin, S. et al. Alterations of Gefitinib Pharmacokinetics by Co-administration of Herbal Medications in Rats. Chin. J. Integr. Med. 24, 460–466 (2018). https://doi.org/10.1007/s11655-017-2907-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11655-017-2907-9