Abstract
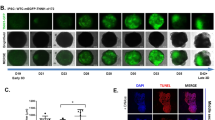
To promote the differentiation of human-induced pluripotent stem cells (hiPSCs) into myocardium through a standard chemically defined and small-molecule-based induction protocol (CDM3), and preliminarily prepare myocardial patches that provide experimental data and theoretical support for further maturation through other in vitro experiments and safety studies in vivo. After resuscitation, culture, and identification of hiPSCs, they were inoculated onto Matrigel-coated polycaprolactone (PCL). After 24 h, cell growth was observed by DAPI under a fluorescence microscope and the stemness of hiPSCs was identified by OCT4 fluorescence. After fixation, scanning electron microscopy was performed to observe the morphology of cells on the patch surface. On days 1, 3, 5, and 7 of culture, cell viability was determined by Cell Counting Kit-8 (CCK-8) assay and a curve was drawn to observe cell growth and proliferation. After co-culture with Matrigel-covered PCL for 24 h, hiPSCs were divided into control and CDM3 groups, and cultured for an additional 6 d. On the eighth day, cell growth was observed by DAPI under a fluorescence microscope, hiPSC stemness was identified by OCT4 fluorescence, and cardiomyocytes were identified by cardiac troponin T (cTnT) and α-actin expression. hiPSCs co-cultured with Matrigel-covered PCL for 24 h emitted green fluorescence indicating OCT4, showing that hiPSCs maintained their stemness on Matrigel-covered PCL scaffolds. DAPI emitted blue fluorescence, indicating that cells grew clonally with uniform cell morphology. Scanning electron microscopy showed that hiPSCs adhered and grew on PCL covered with Matrigel, with clearly visible cell outlines indicating normal morphology. Assessment of cell viability by the CCK-8 method showed that hiPSCs proliferated and grew on PCL scaffolds covered with Matrigel. After 6 d of culture, immunofluorescence showed that control group hiPSCs highly expressed the stem cell marker OCT4 but not myocardial markers cTnT or α-actin. In contrast, notable expression of myocardial markers cTnT and α-actin but not OCT4 occurred in the CDM3 group. hiPSCs can proliferate and grow on PCL scaffolds covered with Matrigel. Under the influence of CDM3, hiPSCs differentiated into cardiomyocyte-like cells, allowing the preliminary preparation of myocardial patches that can provide a better method for clinical treatment of myocardial infarction.







Similar content being viewed by others
Data availability
Not applicable.
References
Anderson JL, Morrow DA (2017) Acute myocardial infarction. N Engl J Med 376(21):2053–2064
Anderson ME, Goldhaber J, Houser SR et al (2014) Embryonic stem cell–derived cardiac myocytes are not ready for human trials. Circ Res 115(3):335–338
Burridge PW, Matsa E, Shukla P et al (2014) Chemically defined generation of human cardiomyocytes. Nat Methods 11(8):855–860
Cahill TJ, Choudhury RP, Riley PR (2017) Heart regeneration and repair after myocardial infarction: translational opportunities for novel therapeutics. Nat Rev Drug Discovery 16(10):699–717
Chong JJH, Yang X, Don CW et al (2014) Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 510(7504):273–277
Csobonyeiova M, Polak S, Nicodemou A et al (2021) iPSCs in modeling and therapy of osteoarthritis. Biomedicines 9(2):186
Gerecht-Nir S, Radisic M, Park H et al (2006) Biophysical regulation during cardiac development and application to tissue engineering. Int J Dev Biol 50(2–3):233–243
Hendrickson T, Mancino C, Whitney L et al (2021) Mimicking cardiac tissue complexity through physical cues: a review on cardiac tissue engineering approaches. Nanomed: Nanotechnol Biol Med 33:102367
Hou X, Ma S, Fan W et al (2022) Chemically defined and small molecules-based generation of sinoatrial node-like cells. Stem Cell Res Ther 13(1):158
Iberite F, Gruppioni E, Ricotti L (2022) Skeletal muscle differentiation of human iPSCs meets bioengineering strategies: perspectives and challenges. NPJ Regen Med 7(1):23
Karagiannis P, Takahashi K, Saito M et al (2019) Induced pluripotent stem cells and their use in human models of disease and development. Physiol Rev 99(1):79–114
Kerr CM, Richards D, Menick DR et al (2021) Multicellular human cardiac organoids transcriptomically model distinct tissue-level features of adult myocardium. Int J Mol Sci 22(16):8482
Laflamme MA, Murry CE (2011) Heart regeneration. Nature 473(7347):326–335
Minter-Dykhouse K, Nelson TJ, Folmes C (2022) Uncoupling of proliferative capacity from developmental stage during directed cardiac differentiation of pluripotent stem cells. Stem Cells Dev 31(17–18):521–528
Nguyen R, Da Won BS, Qiao L et al (2021) Developing liver organoids from induced pluripotent stem cells (iPSCs): an alternative source of organoid generation for liver cancer research. Cancer Lett 508:13–17
Niu H, Mu J, Zhang J et al (2013) Comparative study of three types of polymer materials co-cultured with bone marrow mesenchymal stem cells for use as a myocardial patch in cardiomyocyte regeneration. J Mater Sci - Mater Med 24(6):1535–1542
Rachel K, Pathak S, Moorthi A et al (2020) 5-Azacytidine incorporated polycaprolactone-gelatin nanoscaffold as a potential material for cardiomyocyte differentiation. J Biomater Sci Polym Ed 31(1):123–140
Ramesh S, Govarthanan K, Ostrovidov S et al (2021) Cardiac differentiation of mesenchymal stem cells: impact of biological and chemical inducers. Stem Cell Rev Rep 17(4):1343–1361
Rosenblatt-Velin N, Lepore MG, Cartoni C et al (2005) FGF-2 controls the differentiation of resident cardiac precursors into functional cardiomyocytes. J Clin Investig 115(7):1724–1733
Shijun X, Junsheng M, Jianqun Z et al (2016) In vitro three-dimensional coculturing poly3-hydroxybutyrate-co-3-hydroxyhexanoate with mouse-induced pluripotent stem cells for myocardial patch application. J Biomater Appl 30(8):1273–1282
Siddiqui N, Asawa S, Birru B et al (2018) PCL-based composite scaffold matrices for tissue engineering applications. Mol Biotechnol 60(7):506–532
Sowmya B, Hemavathi AB, Panda PK (2021) Poly (epsilon-caprolactone)-based electrospun nano-featured substrate for tissue engineering applications: a review. Prog Biomater 10(2):91–117
Sridharan D, Palaniappan A, Blackstone BN et al (2021) In situ differentiation of human-induced pluripotent stem cells into functional cardiomyocytes on a coaxial PCL-gelatin nanofibrous scaffold. Mater Sci Eng C 118:111354
Streeter BW, Davis ME 2018 Therapeutic cardiac patches for repairing the myocardium. Cham:Springer International Publishing 1144 1–24
Sutton MG, Sharpe N (2000) Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation 101(25):2981–2988
Tan X, Dai Q, Guo T et al (2018) Efficient generation of transgene- and feeder-free induced pluripotent stem cells from human dental mesenchymal stem cells and their chemically defined differentiation into cardiomyocytes. Biochem Biophys Res Commun 495(4):2490–2497
Van Lent J, Verstraelen P, Asselbergh B et al (2021) Induced pluripotent stem cell-derived motor neurons of CMT type 2 patients reveal progressive mitochondrial dysfunction. Brain 144(8):2471–2485
Virani S, Alonso A, Benjamin E J, et al (2020) Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation 141(9)
Wanjare M, Hou L, Nakayama KH et al (2017) Anisotropic microfibrous scaffolds enhance the organization and function of cardiomyocytes derived from induced pluripotent stem cells. Biomater Sci 5(8):1567–1578
Yu H, Lu K, Zhu J et al (2017) Stem cell therapy for ischemic heart diseases. Br Med Bull 121(1):135–154
Zhang Z, Zhou F, Zheng J, et al (2022) Preparation of myocardial patches from DiI-labeled rat bone marrow mesenchymal stem cells and neonatal rat cardiomyocytes contact co-cultured on polycaprolactone film. Biomed Mater (Bristol) 17(4)
Acknowledgements
We thank Liwen Bianji (Edanz) (www.liwenbianji.cn/) for basic language editing of a draft of this manuscript.
Funding
The authors disclose receipt of the following financial support for the research, authorship, and/or publication of this article: 2018 National Natural Science Foundation (81870181) and 2022 National Natural Science Foundation (82270255).
Author information
Authors and Affiliations
Contributions
DY carried out the molecular genetic studies, participated in the immunoassays, and drafted the manuscript. BP carried out flow cytometry. ZJw participated in the co-culture. YB participated in the design of the study and performed the statistical analysis. MJS and ZF conceived of the study, participated in its design and coordination, helped to draft the manuscript, etc. All of the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
This study was approved by the ethics committee of Beijing Anzhen Hospital, Capital Medical University. The batch number of the ethics approval document is GZR-3–072. The Beijing Anzhen Hospital and national animal care and use guidelines were followed.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dai, Y., Zhou, F., Zheng, J. et al. Effect of CDM3 on co-culture of human-induced pluripotent stem cells with Matrigel-covered polycaprolactone to prepare cardiac patches. In Vitro Cell.Dev.Biol.-Animal 59, 256–263 (2023). https://doi.org/10.1007/s11626-023-00764-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-023-00764-4