Abstract
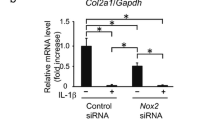
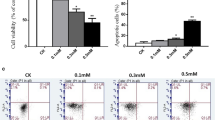
The articular cartilage is an avascular tissue, and oxygen tensions in its superficial and deeper zones are estimated to be 6% and 1%. Degeneration of the articular cartilage begins from the surface zone in osteoarthritis. We previously reported that monocarboxylate transporter-1, a transmembrane transporter for monocarboxylates, played an essential role in the interleukin-1β-induced expression of NADPH oxidase-2, a reactive oxygen species–producing enzyme, and reactive oxygen species–dependent death of mouse chondrogenic ATDC5 cells cultured in a normal condition (20% oxygen). Here, we investigated the effect of oxygen tension on interleukin-1β-induced events described above in ATDC5 cells. Interleukin-1β induced the death of ATDC5 cells under 20% and 6% oxygen but did not under 2% and 1% oxygen. Interleukin-1β induced Mct1 (monocarboxylate transporter-1 gene) and Nox2 (NADPH oxidase-2 gene) mRNAs’ expression under 20% oxygen in 24 h, respectively, but not under 2% oxygen. On the other hand, a 24-h incubation with interleukin-1β upregulated the expression of Nos2 (inducible nitric oxide synthase gene) mRNA irrespective of oxygen tension. Furthermore, inhibition of I-κB kinase suppressed the interleukin-1β-induced expression of Mct1 mRNA in the cells cultured under 20% and 2% oxygen, indicating NF-κB plays an essential role in the induction of the Mct1 gene expression. The results suggest that interleukin-1β induces monocarboxylate transporter-1 in an oxygen tension–dependent manner required for cell death in ATDC5 cells. These results might explain some part of the degenerative process of the articular cartilage, which begins from its superficial zone in the pathogenesis of osteoarthritis.




Similar content being viewed by others
References
Akaike T, Fujii S, Kato A, Akaki-Yoshitake J, Miyamoto Y, Sawa T, Asakawa M, Okamoto S, Suga M, Nagai Y, Maeda H (2000) Viral mutation and molecular evolution accelerated by nitric oxide-induced oxidative stress. FASEB J 14:1447–1454
Alam MS, Akaike T, Okamoto S, Kubota T, Yoshitake J, Sawa T, Miyamoto Y, Tamura F, Maeda H (2002) Host defense role of nitric oxide in murine salmonellosis as a function of its potent antibacterial and antiapoptotic activities. Infect Immunol 70:3130–3142
Barnucz E, Veres G, Hegedüs O, Klein S, Zöller R, Radovits T, Korkmaz S, Horkay F, Merkely B, Karck M, Szabó G (2013) Prolyl-hydroxylase inhibition preserves endothelial cell function in a rat model of vascular ischemia reperfusion injury. J Pharmacol Exp Ther 345:25–31
Borthakur A, Saksena S, Gill RK, Alrefai WA, Ramaswamy K, Dudeja PK (2008) Regulation of monocarboxylate transporter 1 (MCT1) promoter by butyrate in human intestinal epithelial cells: involvement of NF-κB pathway. J Cell Biochem 103:1452–1463
Felmlee MA, Jones RS, Rodriguez-Cruz V, Follman KE, Morris ME (2020) Monocarboxylate transporters (SLC16): function, regulation, and role in health and disease. Pharmacol Rev 72:466–485
Fu B, Xue J, Li Z, Shi X, Jiang B-H, Fang J (2007) Chrysin inhibits expression of hypoxia-inducible factor-1α through reducing hypoxia-inducible factor-1α stability and inhibiting its protein synthesis. Mol Cancer Ther 6:220–226
Funato S, Yasuhara R, Yoshimura K, Miyamoto Y, Kaneko K, Suzawa T, Chikazu D, Mishima K, Baba K, Kamijo R (2017) Extracellular matrix loss in chondrocytes after exposure to interleukin-1β in NADPH oxidase-dependent manner. Cell Tissue Res 368:135–144
Halestrap AP, Wilson MC (2012) The monocarboxylate transporter family – role and regulation. IUBMB Life 64:109–119
Hwang HS, Kim HA (2015) Chondrocyte apoptosis in the pathogenesis of osteoarthritis. Int J Mol Sci 16:26035–26054
Jenei-Lanzi Z, Meurer A, Zaucke F (2019) Interleukin-1β signaling in osteoarthritis – chondrocytes in focus. Cell Signal 53:212–223
Ke Q, Costa M (2006) Hypoxia-inducible factor-1 (HIF-1). Mol Pharmacol 70:1469–1480
Kuwahara H, Miyamoto Y, Akaike T, Kubota T, Sawa T, Okamoto S, Maeda H (2000) Helicobacter pylori urease suppresses bactericidal activity of peroxynitrite via carbon dioxide production. Infect Immunol 68:4378–4383
Miyamoto Y, Akaike T, Alam MS, Inoue K, Hamamoto T, Ikebe N, Yoshitake J, Maeda H (2000) Novel functions of human α1-protease inhibitor after S-nitrosylation: inhibition of cysteine protease and antibacterial activity. Biochem Biophys Res Commun 267:918–923
Miyamoto Y, Akaike T, Yoshida M, Goto S, Horie H, Maeda H (1996) Potentiation of nitric oxide-mediated vasorelaxation by xanthine oxidase inhibitors. Proc Soc Exp Biol Med 211:366–373
Panula HE, Hyttinen MM, Arokoski JPA, Långsjö PA, Kiviranta I, Helminen HJ (1998) Articular cartilage superficial zone collagen birefringence reduced and cartilage thickness increased before surface fibrillation in experimental osteoarthritis. Ann Rheum Dis 57:237–245
Pfandor D, Gelse K (2007) Hypoxia and osteoarthritis: how chondrocytes survive hypoxic environments. Curr Opin Rheumatol 19:457–462
Rousset F, Nguyen MVC, Grange L, Morel F, Lardy B (2013) Heme oxygenase-1 regulates matrix metalloproteinase MMP-1 secretion and chondrocyte cell death via Nox4 NADPH oxidase activity in chondrocytes. PLoS One 8:e66478
Samuvel DJ, Sundararaj KP, Nareika A, Lopes-Virella MF, Huang Y (2009) Lactate boosts TLR4 signaling and NF-κB pathway-mediated gene transcription in macrophages via MD-2 up-regulation. J Immunol 182:2476–2484
Shikhman AR, Brinson DC, Lotz MK (2004) Distinct pathway regulate facilitated glucose transport in human articular chondrocytes during anabolic and catabolic responses. Am J Physiol Endocrinol Metab 286:E980-985
Silver IA (1975) Measurement of pH and ionic composition of pericellular sites. Philos Trans R Soc Lond B Biol Sci 271:261–272
Sun J, Druhan LJ, Zweier JL (2010) Reactive oxygen and nitrogen species regulate inducible nitric oxide synthase function shifting the balance of nitric oxide and superoxide production. Arch Biochem Biophys 494:130–137
Turrens JF (2003) Mitochondrial formation of reactive oxygen species. J Physiol 552:335–344. https://doi.org/10.1113/jphysiol.2003.049478
Winterbourn CC, Kettle AJ, Hampton MB (2016) Reactive oxygen species and neutrophil function. Annu Rev Biochem 85:765–792
Yasuhara R, Miyamoto Y, Akaike T, Akuta T, Nakamura M, Takami M, Morimura N, Yasu K, Kamijo R (2005) Interleukin-1ß induces death in chondrocyte-like ATDC5 cells through mitochondrial dysfunction and energy depletion in a reactive nitrogen and oxygen species-dependent manner. Biochem J 389:315–323. https://doi.org/10.1042/BJ20041996
Yoshimura K, Miyamoto Y, Yasuhara R, Maruyama T, Akiyama T, Yamada A, Takami M, Suzawa T, Tunawaki S, Tachikawa T, Baba K, Kamijo R (2011) Monocarboxylate transporter-1 is required for cell death in mouse chondrocytic ATDC5 cells exposed to interleukin-1ß via late phase activation of nuclear factor kB and expression of phagocyte-type NADPH oxidase. J Biol Chem 286:14744–14752. https://doi.org/10.1074/jbc.M111.221259
Zhou R-P, Dai B-B, Xie Y-Y, Wu X-S, Wang Z-S, Li Y, Wnag Z-Q, Zu S-Q, Ge J-F, Chen F-H (2018) Interleukin-1β and tumor necrosis factor-α augment acidosis-induced rat articular chondrocyte apoptosis via nuclear factor-kappaB-dependent upregulation of ASC1a channel. BBA Mol Basis Dis 1864:162–177
Funding
This work was supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS Kakenhi) (19K18950, 19H03820, 18K19655, 18K09528, 18K09513).
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tanaka, M., Miyamoto, Y., Sasa, K. et al. Low oxygen tension suppresses the death of chondrocyte-like ATDC5 cells induced by interleukin-1ß. In Vitro Cell.Dev.Biol.-Animal 58, 521–528 (2022). https://doi.org/10.1007/s11626-022-00680-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-022-00680-z