Abstract
Background
Microvascular invasion (MVI) relates to poor survival in hepatocellular carcinoma (HCC) patients. In this study, we aim at developing a nomogram for MVI prediction and potential assistance in surgical planning.
Methods
A total of 357 patients were assigned to training (n = 257) and validation (n = 100) cohort. Univariate and multivariate analyses were used to reveal preoperative predictors for MVI. A nomogram incorporating independent predictors was constructed and validated. Disease-free survival was compared between patients, and the potential of the predicted MVI in making surgical procedure was also explored.
Results
Pathological examination confirmed MVI in 140 (39.2%) patients. Imaging features including larger tumor, intra-tumoral artery, tumor type, and higher serum AFP independently correlated with MVI. The nomogram showed desirable performance with an AUROC of 0.803 (95% CI, 0.746–0.860) and 0.814 (95% CI, 0.720–0.908) in the training and validation cohorts, respectively. Good calibration were also revealed by calibration curve in both cohorts. The decision curve analysis indicated that the prediction nomogram was of promising usefulness in clinical work. In addition, survival analysis revealed that patients with positive-predicted MVI suffered a higher risk of early recurrence (P < 0.01). There was no difference in disease-free survival between anatomic or non-anatomic resection in large HCC or small HCC without nomogram-predicted MVI. However, anatomic resection improved disease-free survival in small HCC with nomogram-predicted MVI.
Conclusions
The nomogram obtained desirable results in predicting MVI. Patients with predicted MVI were associated with early recurrence and anatomic resection was recommended for small HCC patients with predicted MVI.




Similar content being viewed by others
References
Bruix J, Reig M, Sherman M. Evidence-Based Diagnosis, Staging, and Treatment of Patients With Hepatocellular Carcinoma. Gastroenterology. 2016; 150: 835–53.
Vitale A, Peck-Radosavljevic M, Giannini EG, Vibert E, Sieghart W, Van Poucke S, Pawlik TM. Personalized treatment of patients with very early hepatocellular carcinoma. J Hepatol. 2017; 66: 412–23.
Zhang X, Li J, Shen F, Lau WY. Significance of presence of microvascular invasion in specimens obtained after surgical treatment of hepatocellular carcinoma. J Gastroenterol Hepatol. 2018; 33: 347–54.
Rodríguez-Perálvarez M, Luong TV, Andreana L, Meyer T, Dhillon AP, Burroughs AK. A Systematic Review of Microvascular Invasion in Hepatocellular Carcinoma: Diagnostic and Prognostic Variability. Ann Surg Oncol. 2012; 20: 325–39.
McHugh PP, Gilbert J, Vera S, Koch A, Ranjan D, Gedaly R. Alpha-fetoprotein and tumour size are associated with microvascular invasion in explanted livers of patients undergoing transplantation with hepatocellular carcinoma. HPB (Oxford). U; 12: 56–61.
Cucchetti A, Piscaglia F, Grigioni AD, Ravaioli M, Cescon M, Zanello M, Grazi GL, Golfieri R, Grigioni WF, Pinna AD. Preoperative prediction of hepatocellular carcinoma tumour grade and micro-vascular invasion by means of artificial neural network: a pilot study. J Hepatol. 2010; 52: 880–8.
Eguchi S, Takatsuki M, Hidaka M, Soyama A, Tomonaga T, Muraoka I, Kanematsu T. Predictor for histological microvascular invasion of hepatocellular carcinoma: a lesson from 229 consecutive cases of curative liver resection. World J Surg. 2010; 34: 1034–8.
Pote N, Cauchy F, Albuquerque M, Voitot H, Belghiti J, Castera L, Puy H, Bedossa P, Paradis V. Performance of PIVKA-II for early hepatocellular carcinoma diagnosis and prediction of microvascular invasion. J Hepatol. 2015; 62: 848–54.
Gouw AS, Balabaud C, Kusano H, Todo S, Ichida T, Kojiro M. Markers for microvascular invasion in hepatocellular carcinoma: where do we stand? Liver Transpl. 2011; 17 Suppl 2: S72–80.
Sterling RK, Wright EC, Morgan TR, Seeff LB, Hoefs JC, Di Bisceglie AM, Dienstag JL, Lok AS. Frequency of elevated hepatocellular carcinoma (HCC) biomarkers in patients with advanced hepatitis C. Am J Gastroenterol. 2012; 107: 64–74.
Lee JM, Yoon JH, Joo I, Woo HS. Recent Advances in CT and MR Imaging for Evaluation of Hepatocellular Carcinoma. Liver Cancer. 2012; 1: 22–40.
Ahn SY, Lee JM, Joo I, Lee ES, Lee SJ, Cheon GJ, Han JK, Choi BI. Prediction of microvascular invasion of hepatocellular carcinoma using gadoxetic acid-enhanced MR and (18)F-FDG PET/CT. Abdom Imaging. 2015; 40: 843–51.
Kim H, Park MS, Choi JY, Park YN, Kim MJ, Kim KS, Choi JS, Han KH, Kim E, Kim KW. Can microvessel invasion of hepatocellular carcinoma be predicted by pre-operative MRI? Eur Radiol. 2009; 19: 1744–51.
Ariizumi S, Kitagawa K, Kotera Y, Takahashi Y, Katagiri S, Kuwatsuru R, Yamamoto M. A non-smooth tumor margin in the hepatobiliary phase of gadoxetic acid disodium (Gd-EOB-DTPA)-enhanced magnetic resonance imaging predicts microscopic portal vein invasion, intrahepatic metastasis, and early recurrence after hepatectomy in patients with hepatocellular carcinoma. J Hepatobiliary Pancreat Sci. 2011; 18: 575–85.
Xu P, Zeng M, Liu K, Shan Y, Xu C, Lin J. Microvascular invasion in small hepatocellular carcinoma: is it predictable with preoperative diffusion-weighted imaging? J Gastroenterol Hepatol. 2014; 29: 330–6.
Suh YJ, Kim MJ, Choi JY, Park MS, Kim KW. Preoperative prediction of the microvascular invasion of hepatocellular carcinoma with diffusion-weighted imaging. Liver Transpl. 2012; 18: 1171–8.
Zhao W, Liu W, Liu H, Yi X, Hou J, Pei Y, Liu H, Feng D, Liu L, Li W. Preoperative prediction of microvascular invasion of hepatocellular carcinoma with IVIM diffusion-weighted MR imaging and Gd-EOB-DTPA-enhanced MR imaging. PLoS One. 2018; 13: e0197488.
Akamatsu N, Cillo U, Cucchetti A, Donadon M, Pinna AD, Torzilli G, Kokudo N. Surgery and Hepatocellular Carcinoma. Liver Cancer. 2016; 6: 44–50.
Shindoh J, Makuuchi M, Matsuyama Y, Mise Y, Arita J, Sakamoto Y, Hasegawa K, Kokudo N. Complete removal of the tumor-bearing portal territory decreases local tumor recurrence and improves disease-specific survival of patients with hepatocellular carcinoma. J Hepatol. 2016; 64: 594–600.
Hasegawa K, Kokudo N, Imamura H, Matsuyama Y, Aoki T, Minagawa M, Sano K, Sugawara Y, Takayama T, Makuuchi M. Prognostic Impact of Anatomic Resection for Hepatocellular Carcinoma. Ann Surg. 2005; 242: 252–9.
Kang CM, Choi GH, Kim DH, Choi SB, Kim KS, Choi JS, Lee WJ. Revisiting the role of nonanatomic resection of small (< or = 4 cm) and single hepatocellular carcinoma in patients with well-preserved liver function. J Surg Res. 2010; 160: 81–9.
Tanaka K, Shimada H, Matsumoto C, Matsuo K, Nagano Y, Endo I, Togo S. Anatomic versus limited nonanatomic resection for solitary hepatocellular carcinoma. Surgery. 2008; 143: 607–15.
Hirokawa F, Kubo S, Nagano H, Nakai T, Kaibori M, Hayashi M, Takemura S, Wada H, Nakata Y, Matsui K, Ishizaki M, Uchiyama K. Do patients with small solitary hepatocellular carcinomas without macroscopically vascular invasion require anatomic resection? Propensity score analysis. Surgery. 2015; 157: 27–36.
Cucchetti A, Qiao GL, Cescon M, Li J, Xia Y, Ercolani G, Shen F, Pinna AD. Anatomic versus nonanatomic resection in cirrhotic patients with early hepatocellular carcinoma. Surgery. 2014; 155: 512–21.
Okusaka T, Okada S, Ueno H, Ikeda M, Shimada K, Yamamoto J, Kosuge T, Yamasaki S, Fukushima N, Sakamoto M. Satellite lesions in patients with small hepatocellular carcinoma with reference to clinicopathologic features. Cancer. 2002; 95: 1931–7.
Cong WM, Bu H, Chen J, Dong H, Zhu YY, Feng LH, Chen J, Guideline C. Practice guidelines for the pathological diagnosis of primary liver cancer: 2015 update. World J Gastroenterol. 2016; 22: 9279–87.
Min JH, Kim YK, Lim S, Jeong WK, Choi D, Lee WJ. Prediction of microvascular invasion of hepatocellular carcinomas with gadoxetic acid-enhanced MR imaging: Impact of intra-tumoral fat detected on chemical-shift images. Eur J Radiol. 2015; 84: 1036–43.
Lee S, Kim SH, Lee JE, Sinn DH, Park CK. Preoperative gadoxetic acid-enhanced MRI for predicting microvascular invasion in patients with single hepatocellular carcinoma. J Hepatol. 2017; 67: 526–34.
Wang WT, Yang L, Yang ZX, Hu XX, Ding Y, Yan X, Fu CX, Grimm R, Zeng MS, Rao SX. Assessment of Microvascular Invasion of Hepatocellular Carcinoma with Diffusion Kurtosis Imaging. Radiology. 2018; 286: 571–80.
Okamura S, Sumie S, Tonan T, Nakano M, Satani M, Shimose S, Shirono T, Iwamoto H, Aino H, Niizeki T, Tajiri N, Kuromatsu R, Okuda K, Nakashima O, Torimura T. Diffusion-weighted magnetic resonance imaging predicts malignant potential in small hepatocellular carcinoma. Dig Liver Dis. 2016; 48: 945–52.
Sakata J, Shirai Y, Wakai T, Kaneko K, Nagahashi M, Hatakeyama K. Preoperative predictors of vascular invasion in hepatocellular carcinoma. Eur J Surg Oncol. 2008; 34: 900–5.
Lee J, Lee J, Kim S, Baek J, Yun S, Kim K, Han J, Choi B. Enhancement patterns of hepatocellular carcinomas on multiphasicmultidetector row CT: comparison with pathological differentiation. Br J Radiol. 2012; 85: e573–83.
Segal E, Sirlin CB, Ooi C, Adler AS, Gollub J, Chen X, Chan BK, Matcuk GR, Barry CT, Chang HY, Kuo MD. Decoding global gene expression programs in liver cancer by noninvasive imaging. Nat Biotechnol. 2007; 25: 675–80.
Oriyama T, Yamanaka N, Fujimoto J, Ichikawa N, Okamoto E. Progression of hepatocellular carcinoma as reflected by nuclear DNA ploidy and cellular differentiation. J Hepatol. 1998; 28: 142–9.
Kanai T, Hirohashi S, Upton M, Noguchi M, Kishi K, Makuuchi M, Yamasaki S, Hasegawa H, Takayasu K, Moriyama N. Pathology of small hepatocellular carcinoma. A proposal for a new gross classification. Cancer. 1987; 60: 810–9.
Chou CT, Chen RC, Lee CW, Ko CJ, Wu HK, Chen YL. Prediction of microvascular invasion of hepatocellular carcinoma by pre-operative CT imaging. Br J Radiol. 2012; 85: 778–83.
Chou CT, Chen RC, Lin WC, Ko CJ, Chen CB, Chen YL. Prediction of microvascular invasion of hepatocellular carcinoma: preoperative CT and histopathologic correlation. AJR Am J Roentgenol. 2014; 203: W253–9.
Wang L, Wang W, Yao X, Rong W, Wu F, Chen B, Liu M, Lin S, Liu Y, Wu J. Postoperative adjuvant radiotherapy is associated with improved survival in hepatocellular carcinoma with microvascular invasion. Oncotarget. 2017; 8: 79971–81.
Wang Z, Ren Z, Chen Y, Hu J, Yang G, Yu L, Yang X, Huang A, Zhang X, Zhou S, Sun H, Wang Y, Ge N, Xu X, Tang Z, Lau W, Fan J, Wang J, Zhou J. Adjuvant Transarterial Chemoembolization for HBV-Related Hepatocellular Carcinoma After Resection: A Randomized Controlled Study. Clin Cancer Res. 2018; 24: 2074–81.
Donat M, Alonso S, Pereira F, Ferrero E, Carrion L, Acin-Gandara D, Moreno E. Impact of Histological Factors of Hepatocellular Carcinoma on the Outcome of Liver Transplantation. Transplant Proc. 2016; 48: 1968–77.
Acknowledgements
We gratefully acknowledge Changfa Xia for the assistance in statistical analysis, Zhuo Li and Bo Zheng for reviewing the pathological results, and Kaijing Liu for revising the manuscript.
Author information
Authors and Affiliations
Contributions
Study concept and design: Liming Wang, Jianxiong Wu, Weiqi Rong
Clinical data acquisition: Shengtao Lin, Yunhe Liu, Yiling Zheng
Patient follow-up: Shengtao Lin, Yunhe Liu
Imaging feature evaluation: Feng Ye, Ying Song
Statistical analysis: Shengtao Lin, Fan Wu, Feng Ye, Tana Siqi
Drafting of the manuscript: Shengtao Lin, Yiling Zheng, Liming Wang
Study supervision: Liming Wang, Jianxiong Wu
Critical revision of the manuscript: all authors
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Figure S1
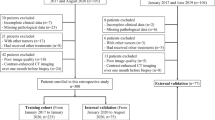
Flow chart of the study population. HCC: Hepatocellular Carcinoma, MR: Magnetic Resonance, TACE: Transhepatic Arterial Chemotherapy And Embolization, RFA: Radiofrequency Ablation. (PNG 491 kb)
Figure S2
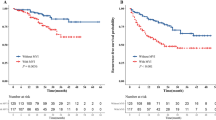
Disease-free survival between HCC patients. Kaplan-Meier survival curves and the Log-Rank Test compared the disease-free survival in patients with the actual presence or absence of MVI (A, P<0.01) and patients with the nomogram predicted presence or absence of MVI (B, P<0.01). (PNG 159 kb)
Figure S3
Disease-free survival in HCC patients received different surgical procedures. Kaplan-Meier survival curves and the Log-Rank Test compared the disease-free survival between different surgical procedures in the entire cohort (A, P=0.26), the patients with large HCCs (>5cm) (B, P=0.97), the patients with small HCCs (≤5cm) (C, P=0.09). (PNG 288 kb)
Table S1
(DOCX 12 kb)
Rights and permissions
About this article
Cite this article
Lin, S., Ye, F., Rong, W. et al. Nomogram to Assist in Surgical Plan for Hepatocellular Carcinoma: a Prediction Model for Microvascular Invasion. J Gastrointest Surg 23, 2372–2382 (2019). https://doi.org/10.1007/s11605-019-04140-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-019-04140-0