Abstract
Purpose
Parkinson’s disease (PD) is caused by a selective degeneration of dopamine neurons. The relationship between dopamine transporter (DAT) density and gray matter volume has been unclear. Here we investigated the voxelwise correlation between gray matter volume and DAT binding measured by 123I-N-ω-fluoropropyl-2β-carboxymethoxy-3β-(4-iodophenyl)nortropane (123I-FP-CIT) single-photon emission computed tomography (SPECT; DaTscan™ imaging) in PD.
Materials and methods
Thirty-one male patients with PD were examined with MRI and DaTscan. To measure nigrostriatal dopaminergic degeneration in PD, the specific binding ratio (SBR) of the striatum was obtained by DaTscan. Voxel-based morphometry (VBM) of 3D T1-weighted images was used to evaluate the relationships between the regional gray matter volume and the SBR in the striatum.
Results
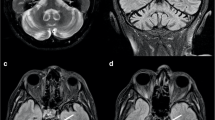
There were significant positive correlations between the SBR and the gray matter volume in the right pulvinar and posterior middle temporal gyrus and a trend level in the left pulvinar, all of which are associated with the second visual pathway.
Conclusion
The nigrostriatal dopaminergic degeneration might affect the secondary visual pathway, leading to visual dysfunctions in PD.

Similar content being viewed by others
References
Jankovic J. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79(4):368–76. doi:10.1136/jnnp.2007.131045.
Politis M. Neuroimaging in Parkinson disease: from research setting to clinical practice. Nat Rev Neurol. 2014;10(12):708–22. doi:10.1038/nrneurol.2014.205.
Suwijn SR, van Boheemen CJ, de Haan RJ, Tissingh G, Booij J, de Bie RM. The diagnostic accuracy of dopamine transporter SPECT imaging to detect nigrostriatal cell loss in patients with Parkinson’s disease or clinically uncertain parkinsonism: a systematic review. EJNMMI Res. 2015;5:12. doi:10.1186/s13550-015-0087-1.
Cummings JL, Henchcliffe C, Schaier S, Simuni T, Waxman A, Kemp P. The role of dopaminergic imaging in patients with symptoms of dopaminergic system neurodegeneration. Brain. 2011;134(Pt 11):3146–66. doi:10.1093/brain/awr177.
Gerasimou G, Costa DC, Papanastasiou E, Bostanjiopoulou S, Arnaoutoglou M, Moralidis E, et al. SPECT study with I-123-ioflupane (DaTSCAN) in patients with essential tremor. Is there any correlation with Parkinson’s disease? Ann Nucl Med. 2012;26(4):337–44. doi:10.1007/s12149-012-0577-4.
Burton EJ, McKeith IG, Burn DJ, Williams ED, O’Brien JT. Cerebral atrophy in Parkinson’s disease with and without dementia: a comparison with Alzheimer’s disease, dementia with Lewy bodies and controls. Brain. 2004;127(Pt 4):791–800.
Summerfield C, Junqué C, Tolosa E, Salgado-Pineda P, Gómez-Ansón B, Martí MJ, et al. Structural brain changes in Parkinson disease with dementia: a voxel-based morphometry study. Arch Neurol. 2005;62(2):281–5.
Beyer MK, Janvin CC, Larsen JP, Aarsland D. A magnetic resonance imaging study of patients with Parkinson’s disease with mild cognitive impairment and dementia using voxel-based morphometry. J Neurol Neurosurg Psychiatr. 2007;78(3):254–9.
Melzer TR, Watts R, MacAskill MR, Pitcher TL, Livingston L, Keenan RJ, et al. Grey matter atrophy in cognitively impaired Parkinson’s disease. J Neurol Neurosurg Psychiatr. 2012;83(2):188–94. doi:10.1136/jnnp-2011-300828.
Compta Y, Ibarretxe-Bilbao N, Pereira JB, Junqué C, Bargalló N, Tolosa E, et al. Grey matter volume correlates of cerebrospinal markers of Alzheimer-pathology in Parkinson’s disease and related dementia. Parkinsonism Relat Disord. 2012;18(8):941–7. doi:10.1016/2012.04.028 (j.parkreldis.).
Sanchez-Castaneda C, Rene R, Ramirez-Ruiz B, Campdelacreu J, Gascon J, Falcon C, et al. Correlations between gray matter reductions and cognitive deficits in dementia with Lewy Bodies and Parkinson’s disease with dementia. Mov Disord. 2009;24(12):1740–6. doi:10.1002/mds.22488.
Huang C, Mattis P, Tang C, Perrine K, Carbon M, Eidelberg D. Metabolic brain networks associated with cognitive function in Parkinson’s disease. Neuroimage. 2007;34(2):714–23.
Huang C, Mattis P, Perrine K, Brown N, Dhawan V, Eidelberg D. Metabolic abnormalities associated with mild cognitive impairment in Parkinson disease. Neurology. 2008;70(16 Pt 2):1470–7.
Nobili F, Arnaldi D, Campus C, Ferrara M, De Carli F, Brugnolo A, et al. Brain perfusion correlates of cognitive and nigrostriatal functions in de novo Parkinson’s disease. Eur J Nucl Med Mol Imaging. 2011;38(12):2209–18.
Choi H, Cheon GJ, Kim HJ, Choi SH, Kim YI, Kang KW, et al. Gray matter correlates of dopaminergic degeneration in Parkinson’s Disease: a hybrid PET/MR study using (18) F-FP-CIT. Hum Brain Mapp. 2016;37(5):1710–21.
Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatr. 1992;55(3):181–4.
Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17(5):427–42.
Goetz CG, Poewe W, Rascol O, Sampaio C, Stebbins GT, Counsell C, et al. Movement Disorder Society Task Force report on the Hoehn and Yahr Staging scale: status and recommendations. Mov Disord. 2004;19(9):1020–8.
Tossici-Bolt L, Hoffmann SM, Kemp PM, Mehta RL, Fleming JS. Quantification of [123I]FP-CIT SPECT brain images: an accurate technique for measurement of the specific binding ratio. Eur J Nucl Med Mol Imaging. 2006;33(12):1491–9.
Robinson DL, Petersen SE. The pulvinar and visual salience. Trends Neurosci. 1992;15(4):127–32.
Komura Y, Nikkuni A, Hirashima N, Uetake T, Miyamoto A. Responses of pulvinar neurons reflect a subject’s confidence in visual categorization. Nat Neurosci. 2013;16(6):749–55.
Danckert J, Rossetti Y. Blindsight in action: what can the different sub-types of blindsight tell us about the control of visually guided actions? Neurosci Biobehav Rev. 2005;29(7):1035–46.
Diederich NJ, Stebbins G, Schiltz C, Goetz CG. Are patients with Parkinson’s disease blind to blindsight? Brain. 2014;137(Pt 6):1838–49. doi:10.1093/brain/awu094.
Ku J, Kim JJ, Jung YC, Park IH, Lee H, Han K, et al. Brain mechanisms involved in processing unreal perceptions. Neuroimage. 2008;43(4):793–800. doi:10.1016/j.neuroimage.2008.08.011.
Boecker H, Ceballos-Baumann AO, Volk D, Conrad B, Forstl H, Haussermann P. Metabolic alterations in patients with Parkinson disease and visual hallucinations. Arch Neurol. 2007;64(7):984–8.
Zeki S, Watson JD, Lueck CJ, Friston KJ, Kennard C, Frackowiak RS. A direct demonstration of functional specialization in human visual cortex. J Neurosci. 1991;11(3):641–9.
Tootell RB, Reppas JB, Kwong KK, Malach R, Born RT, Brady TJ, et al. Functional analysis of human MT and related visual cortical area using magnetic resonance imaging. J Neurosci. 1995;15(4):3215–30.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Informed consent
This retrospective study was approved by the institutional review board at the National Center of Neurology and Psychiatry Hospital, and the need for patient informed consent was waived.
Ethical Statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required. This article does not contain any studies with animals performed by any of the authors.
Conflict of interest
This study was commissioned by Nihon Medi-Physics Co., Ltd., Hyogo, Japan (grant no. 174). Author Noriko Sato has received research grants from Nihon Medi-Physics Co.
About this article
Cite this article
Maekawa, T., Sato, N., Ota, M. et al. Correlations between dopamine transporter density measured by 123I-FP-CIT SPECT and regional gray matter volume in Parkinson’s disease. Jpn J Radiol 35, 755–759 (2017). https://doi.org/10.1007/s11604-017-0694-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-017-0694-z