Abstract
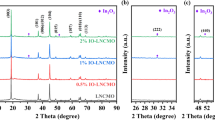
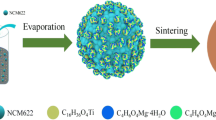
LiNi0.5Mn1.5O4 (LNMO) cathodes always suffer from severe capacity fading because of the oxidization of electrolyte and the dissolution of transition metal. Surface modification has been widely recognized as one of the most effective approaches. Herein, a uniform Fe2O3 layer was coated on LNMO via a simple solid-state method with Prussian blue as precursor. The SEM, TEM, XPS, and XRD results show that the Fe2O3 layer was effectively coated on the surface of LNMO. Fe2O3 layer not only increased the specific capacity of Li/LNMO@Fe2O3–0.65% (more than 120 mAh g−1, 2 C) but also improved the capacity retention (more than 90% after 300 cycles at 25 °C). While those of LNMO are about 94 mAh g−1 and 74%, respectively. These are owing to the successful suppression of side reactions and the dissolution of Mn and Ni. Therefore, this long-term cycling coated material is a promising candidate for high-energy lithium-ion batteries.












Similar content being viewed by others
References
Yi T-F, Mei J, Zhu Y-R (2016) Key strategies for enhancing the cycling stability and rate capacity of LiNi0.5Mn1.5O4 as high-voltage cathode materials for high power lithium-ion batteries. J Power Sources 316:85–105. https://doi.org/10.1016/j.jpowsour.2016.03.070
Kozawa T, Murakami T, Naito M (2016) Insertion of lattice strains into ordered LiNi0.5Mn1.5O4 spinel by mechanical stress: a comparison of perfect versus imperfect structures as a cathode for Li-ion batteries. J Power Sources 320:120–126. https://doi.org/10.1016/j.jpowsour.2016.04.086
Höweling A, Stenzel D, Gesswein H, Kaus M, Indris S, Bergfeldt T, Binder JR (2016) Variations in structure and electrochemistry of iron- and titanium-doped lithium nickel manganese oxyfluoride spinels. J Power Sources 315:269–276. https://doi.org/10.1016/j.jpowsour.2016.03.023
Yoon J, Jeong M, Bae IT, Nam K-W, Yoon W-S (2017) Zr-doping effect on the capacity retention of LiNi0.5Mn1.5O4–δ cycled between 5.0 and 1.0 V: in situ synchrotron X-ray diffraction study. J Power Sources 368:1–10. https://doi.org/10.1016/j.jpowsour.2017.09.056
Zheng X, Liu W, Qu Q, Shi Q, Zheng H, Huang Y (2018) Effectively stabilizing 5 V spinel LiNi0.5Mn1.5O4 cathode in organic electrolyte by polyvinylidene fluoride coating. Appl Surf Sci 455:349–356. https://doi.org/10.1016/j.apsusc.2018.05.151
Hu E, Bak S-M, Liu Y, Liu J, Yu X, Zhou Y-N, Zhou J, Khalifah P, Ariyoshi K, Nam K-W, Yang X-Q (2016) Utilizing environmental friendly iron as a substitution element in spinel structured cathode materials for safer high energy lithium-ion batteries. Adv Energy Mater 6(3):1501662. https://doi.org/10.1002/aenm.201501662
Deng H, Nie P, Luo H, Zhang Y, Wang J, Zhang X (2014) Highly enhanced lithium storage capability of LiNi0.5Mn1.5O4 by coating with Li2TiO3 for Li-ion batteries. J Mater Chem A 2(43):18256–18262. https://doi.org/10.1039/c4ta03802a
Chemelewski KR, Shin DW, Li W, Manthiram A (2013) Octahedral and truncated high-voltage spinel cathodes: the role of morphology and surface planes in electrochemical properties. J Mater Chem A 1(10):3347. https://doi.org/10.1039/c3ta00682d
Guo J, Qin X, Zong B, Zhou M, Wu W, Wang L (2017) Effects of lithium excess amount on the microstructure and electrochemical properties of LiNi0.5Mn1.5O4 cathode material. Ionics 24(8):2241–2250. https://doi.org/10.1007/s11581-017-2374-5
Wang G, Wen W, Chen S, Yu R, Wang X, Yang X (2016) Improving the electrochemical performances of spherical LiNi0.5Mn1.5O4 by Fe2O3 surface coating for lithium-ion batteries. Electrochim Acta 212:791–799. https://doi.org/10.1016/j.electacta.2016.07.025
Zhang Y-H, Zhao Q, Cong L-N, Bao S-D, Xie H-M, Sun L-Q (2016) Fabrication of CNTs/MnO2 composite as a wrapping layer for surface modification of Cr-doped LiNi0.5Mn1.5O4 for lithium ion batteries. RSC Adv 6(91):88719–88726. https://doi.org/10.1039/c6ra18764a
Li Y, Zhang Q, Xu T, Wang D, Pan D, Zhao H, Bai Y (2018) LaF3 nanolayer surface modified spinel LiNi0.5Mn1.5O4 cathode material for advanced lithium-ion batteries. Ceram Int 44(4):4058–4066. https://doi.org/10.1016/j.ceramint.2017.11.203
Piao JY, Liu XC, Wu J, Yang W, Wei Z, Ma J, Duan SY, Lin XJ, Xu YS, Cao AM, Wan LJ (2018) Construction of uniform cobalt-based nanoshells and its potential for improving Li-ion battery performance. ACS Appl Mater Interfaces 10(27):22896–22901. https://doi.org/10.1021/acsami.8b08528
Li X, Zhang K, Mitlin D, Yang Z, Wang M, Tang Y, Jiang F, Du Y, Zheng J (2018) Fundamental insight into Zr modification of Li- and Mn-rich cathodes: combined transmission electron microscopy and electrochemical impedance spectroscopy study. Chem Mater 30(8):2566–2573. https://doi.org/10.1021/acs.chemmater.7b04861
Wang J, Nie P, Xu G, Jiang J, Wu Y, Fu R, Dou H, Zhang X (2018) High-voltage LiNi0.45Cr0.1Mn1.45O4 cathode with superlong cycle performance for wide temperature lithium-ion batteries. Adv Funct Mater 28(4):1704808. https://doi.org/10.1002/adfm.201704808
Zhou L, Zhao D, Lou XD (2012) LiNi0.5Mn1.5O4 hollow structures as high-performance cathodes for lithium-ion batteries. Angew Chem Int Ed 51(1):239–241. https://doi.org/10.1002/anie.201106998
Wang L, Chen D, Wang J, Liu G, Wu W, Liang G (2015) Improved structural and electrochemical performances of LiNi0.5Mn1.5O4 cathode materials by Cr3+ and/or Ti4+ doping. RSC Adv 5(121):99856–99865. https://doi.org/10.1039/c5ra20003b
Fang X, Ge M, Rong J, Zhou C (2013) Graphene-oxide-coated LiNi0.5Mn1.5O4 as high voltage cathode for lithium ion batteries with high energy density and long cycle life. J Mater Chem A 1(12):4083–4088. https://doi.org/10.1039/c3ta01534c
Xu T, Li Y, Wang D, Wu M, Pan D, Zhao H, Bai Y (2018) Enhanced electrochemical performance of LiNi0.5Mn1.5O4 cathode material by YPO4 surface modification. ACS Sustain Chem Eng 6(5):5818–5825. https://doi.org/10.1021/acssuschemeng.7b03935
Hao Q, Xu C, Jia S, Zhao X (2013) Improving the cycling stability of LiCoO2 at 4.5 V through surface modification by Fe2O3 coating. Electrochim Acta 113:439–445. https://doi.org/10.1016/j.electacta.2013.09.105
Ji Y, Zhang Z, Gao M, Li Y, Matthew MDJ, Yang Y (2015) Electrochemical behavior of suberonitrile as a high-potential electrolyte additive and co-solvent for Li[Li0.2Mn0.56Ni0.16Co0.08]O2 cathode material. J Electrochem Soc 162(4):A774–A780. https://doi.org/10.1149/2.1001504jes
Zhi H, Xing L, Zheng X, Xu K, Li W (2017) Understanding how nitriles stabilize electrolyte/electrode interface at high voltage. J Phys Chem Lett 8(24):6048–6052. https://doi.org/10.1021/acs.jpclett.7b02734
Ding Y, Deng B, Wang H, Li X, Chen T, Yan X, Wan Q, Qu M, Peng G (2019) Improved electrochemical performances of LiNi0.6Co0.2Mn0.2O2 cathode material by reducing lithium residues with the coating of Prussian blue. J Alloys Compd 774:451–460. https://doi.org/10.1016/j.jallcom.2018.09.286
Kwon Y, Lee Y, Kim SO, Kim HS, Kim KJ, Byun D, Choi W (2018) Conducting polymer coating on a high-voltage cathode based on soft chemistry approach toward improving battery performance. ACS Appl Mater Interfaces 10(35):29457–29466. https://doi.org/10.1021/acsami.8b08200
Zhao R, Li L, Xu T, Wang D, Pan D, He G, Zhao H, Bai Y (2019) One-step integrated surface modification to build a stable interface on high-voltage cathode for lithium-ion batteries. ACS Appl Mater Interfaces 11:16233–16242. https://doi.org/10.1021/acsami.9b02996
Pang WK, Lin H-F, Peterson VK, Lu C-Z, Liu C-E, Liao S-C, Chen J-M (2017) Enhanced rate-capability and cycling-stability of 5 V SiO2- and polyimide-coated cation ordered LiNi0.5Mn1.5O4 lithium-ion battery positive electrodes. J Phys Chem C 121(7):3680–3689. https://doi.org/10.1021/acs.jpcc.6b10743
Wang H, Ge W, Li W, Wang F, Liu W, Qu MZ, Peng G (2016) Facile fabrication of ethoxy-functional polysiloxane wrapped LiNi0.6Co0.2Mn0.2O2 cathode with improved cycling performance for rechargeable Li-ion battery. ACS Appl Mater Interfaces 8(28):18439–18449. https://doi.org/10.1021/acsami.6b04644
Ming H, Ming J, Oh SM, Tian S, Zhou Q, Huang H, Sun YK, Zheng J (2014) Surfactant-assisted synthesis of Fe2O3 nanoparticles and F-doped carbon modification toward an improved Fe3O4@CFx/LiNi0.5Mn1.5O4 battery. ACS Appl Mater Interfaces 6(17):15499–15509. https://doi.org/10.1021/am504144d
Zhu W, Liu D, Trottier J, Gagnon C, Howe J, Mauger A, Julien CM, Zaghib K (2015) In-situ Raman spectroscopic investigation of LiMn1.45Ni0.45M0.1O4 (M=Cr, Co) 5 V cathode materials. J Power Sources 298:341–348. https://doi.org/10.1016/j.jpowsour.2015.07.083
Luo Y, Lu T, Zhang Y, Yan L, Mao SS, Xie J (2017) Surface-segregated, high-voltage spinel lithium-ion battery cathode material LiNi0.5Mn1.5O4 cathodes by aluminium doping with improved high-rate cyclability. J Alloys Compd 703:289–297. https://doi.org/10.1016/j.jallcom.2017.01.248
Chen Z, Zhao R, Li A, Hu H, Liang G, Lan W, Cao Z, Chen H (2015) Polyhedral ordered LiNi0.5Mn1.5O4 spinel with excellent electrochemical properties in extreme conditions. J Power Sources 274:265–273. https://doi.org/10.1016/j.jpowsour.2014.10.073
Deng M-M, Zou B-K, Shao Y, Tang Z-F, Chen C-H (2017) Comparative study of the electrochemical properties of LiNi0.5Mn1.5O4 doped by bivalent ions (Cu2+, Mg2+, and Zn2+). J Solid State Electrochem 21(6):1733–1742. https://doi.org/10.1007/s10008-017-3545-z
Han Y-K, Jung J, Yu S, Lee H (2009) Understanding the characteristics of high-voltage additives in Li-ion batteries: solvent effects. J Power Sources 187(2):581–585. https://doi.org/10.1016/j.jpowsour.2008.10.137
Leggesse EG, Wei T-Y, Nachimuthu S, Jiang JC (2016) Theoretical study of the reductive decomposition of vinylethylene sulfite as an additive in lithium ion battery. J Chin Artic Chem Soc 63(6):480–487. https://doi.org/10.1002/jccs.201600076
Wang H, Sun D, Li X, Ge W, Deng B, Qu M, Peng G (2017) Alternative multifunctional cyclic organosilicon as an efficient electrolyte additive for high performance lithium-ion batteries. Electrochim Acta 254:112–122. https://doi.org/10.1016/j.electacta.2017.09.111
Funding
This work was supported by the CAS “light of West China” Program (Grant No. 1113-39).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, X., Zhang, Y., Qiao, Y. et al. Improving electrochemical performances of LiNi0.5Mn1.5O4 by Fe2O3 coating with Prussian blue as precursor. Ionics 27, 973–981 (2021). https://doi.org/10.1007/s11581-020-03463-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-020-03463-2