Abstract
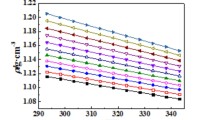
The effects of water addition and temperature on some physicochemical properties of room temperature ionic liquids containing chromium chloride, choline chloride and water in the molar ratio of 1:2.5:x (where x = 6, 9, 12, 15 or 18) have been studied. The density, viscosity, surface tension and conductivity of the liquid mixtures were measured for the temperature range of 25 to 80 °C. Increasing both water content and temperature resulted in decreasing density, surface tension and viscosity and increasing electrical conductivity. The average void radii (hole sizes) for the liquid systems under study were calculated; they were in the range of 1.21 to 1.82 Å. The average hole size was stated to grow with increasing both temperature and water content in the mixture. The variation of the average void radii correlates with the change in viscosity and conductivity. The activation energies of viscous flow and conductivity diminishes with increasing water content in the liquid mixture. There is a strong linear correlation between conductivity and fluidity which indicates that the conductivity of the ionic liquid mixtures is generally controlled by the ionic mobility. A moderate viscosity and higher conductivity of the Cr(III)-containing ionic liquids with extra-water addition (at x > 9) make them suitable for the development of chromium electrodeposition processes.








Similar content being viewed by others
Notes
Let us emphasize that x in our study denominates the total amount of water in the system, both originating from hexahydrate salt CrCl3·6H2O and specially added to the mixture. Thus, the value x = 6 corresponds to the system without extra water. As follows from a simple calculation, the findings described in work [15] refer to x ≈ 14.55.
Note that the measurements of conductivity for the systems CrCl3 + 2.5ChCl + xH2O have been carried out in work [17] only at the temperature value of 60 °C. For comparison, the conductivity of the system with x = 12 is about 2.4 Ω−1 m−1 according to Ref. [17], whereas we obtained σ = 2.158 Ω−1 m−1 for the same system and at the same temperature. Thus, the discrepancy is ca. 10 %.
References
Smith EL, Abbott AP, Ryder KS (2014) Deep eutectic solvents (DESs) and their applications. Chem Rev 114:11060–11082
Zhang Q, De Oliveira Vigier K, Royer S, Jérôme F (2012) Deep eutectic solvents: syntheses, properties and applications. Chem Soc Rev 41:7108–7146
Endres F, MacFarlane D, Abbott A (2008) Electrodeposition from ionic liquids. Wiley VCH
Abbott AP, Capper G, Davies DL, Rasheed RK, Tambyrajah V (2003) Novel solvent properties of choline chloride/urea mixtures. Chem Commun:70–71
Paiva A, Craveiro R, Aroso I, Martins M, Reis RL, Duarte ARC (2014) Natural deep eutectic solvents—solvents for the twenty-first century. ACS Sustain Chem Eng 2:1063–1071
Abbott AP, McKenzie KJ (2006) Application of ionic liquids to the electrodeposition of metals. Phys Chem Chem Phys 8:4265–4279
Abbott AP, Ryder KS, König U (2008) Electrofinishing of metals using eutectic based ionic liquids. Trans Inst Met Finish 86:196–204
Abbott AP, El Ttaib K, Ryder KS, Smith EL (2008) Electrodeposition of nickel using eutectic based ionic liquids. Trans Inst Met Finish 86:234–240
Ghareh Bagh FS, Shahbaz K, Mjalli FS, Hashim MA, AlNashef IM (2015) Zinc (II) chloride-based deep eutectic solvents for application as electrolytes: preparation and characterization. J Mol Liq 204:76–83
Abbott AP, Ballantyne A, Harris RC, Juma JA, Ryder KS, Forrest G (2015) A comparative study of nickel electrodeposition using deep eutectic solvents and aqueous solutions. Electrochim Acta 176:718–726
Protsenko VS, Danilov FI (2014) Chromium electroplating from trivalent chromium baths as an environmentally friendly alternative to hazardous hexavalent chromium baths: comparative study on advantages and disadvantages. Clean Techn Environ Policy 16:1201–1206
Protsenko V, Danilov F (2009) Kinetics and mechanism of chromium electrodeposition from formate and oxalate solutions of Cr(III) compounds. Electrochim Acta 54:5666–5672
Abbott AP, Capper G, Davies DL, Rasheed RK (2004) Ionic liquid analogues formed from hydrated metal salts. Chem Eur J 10:3769–3774
Abbott AP, Al-Barzinjy AA, Abbott PD, Frish G, Harris RC, Hartley J (2014) Ryder K.S., speciation, physical and electrolytic properties of eutectic mixtures based on CrCl3·6H2O and urea. Phys Chem Chem Phys 16:9047–9055
Ferreira ESC, Pereira CM, Silva AF (2013) Electrochemical studies of metallic chromium electrodeposition from a Cr(III) bath. J Electroanal Chem 707:52–58
Mares ML, Ciocirlan O, Cojocaru A, Anicai L (2013) Physico-chemical and electrochemical studies in choline chloride based ionic liquid analogues containing trivalent chromium chloride. Revista de Chimie (Bucharest) 64:815–824
McCalman DC, Sun L, Zhang Y, Brennecke JF, Maginn EJ, Schneider WF (2015) Speciation, conductivities, diffusivities, and electrochemical reduction as a function of water content in mixtures of hydrated chromium chloride/choline chloride. J Phys Chem B 119:6018–6023
Shah D, Mjalli FS (2014) Effect of water on the thermo-physical properties of reline: an experimental and molecular simulation based approach. Phys Chem Chem Phys 16:23900–23907
Protsenko VS, Kityk AA, Shaiderov DA, Danilov FI (2015) Effect of water content on physicochemical properties and electrochemical behavior of ionic liquids containing choline chloride, ethylene glycol and hydrated nickel chloride. J Mol Liq 212:716–722
Bobrova LS, Danilov FI, Protsenko VS (2016) Effects of temperature and water content on physicochemical properties of ionic liquids containing CrCl3·xH2O and choline chloride. J Mol Liq 223:48–53
Mjalli FS, Naser J, Jibril B, Alizadeh V, Gano Z (2014) Tetrabutylammonium chloride based ionic liquid analogues and their physical properties. J Chem Eng Data 59:2242–2251
Abbott AP (2004) Application of hole theory to the viscosity of ionic and molecular liquids. ChemPhysChem 5:1242–1246
Abbott AP, Barron JC, Ryder KS, Wilson D (2007) Eutectic-based ionic liquids with metal-containing anions and cations. Chem Eur J 13:6495–6501
Bockris JO'M, Reddy AKN (2002) Modern electrochemistry, vol. 1 (Ionics). Kluwer Academic Publishers, New York
Abbott AP, Boothby D, Capper G, Davies DL, Rasheed RK (2004) Deep eutectic solvents formed between choline chloride and carboxylic acids: versatile alternatives to ionic liquids. J Am Chem Soc 126:9142–9147
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 129 kb)
Rights and permissions
About this article
Cite this article
Protsenko, V.S., Bobrova, L.S. & Danilov, F.I. Physicochemical properties of ionic liquid mixtures containing choline chloride, chromium (III) chloride and water: effects of temperature and water content. Ionics 23, 637–643 (2017). https://doi.org/10.1007/s11581-016-1826-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-016-1826-7