Abstract
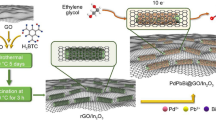
Direct methanol fuel cells (DMFCs) have recently become the research hotspot, due to their high energy-conversion efficiency, low operating temperature, and low exhaust emission. In this study, PtCo nanoalloys supported on reduced graphene oxide (RGO) were successfully synthesized via a one-step reduction method, carried out by coreduction of PtCl6 2−, Co2+ and graphene oxide (GO) in ethylene glycol. Fourier-transform infrared spectra, X-ray diffraction, energy dispersive X-ray spectroscopy, transmission electron microscopy, and X-ray photoelectron spectroscopy techniques were used to study microstructure, composition, particle size, and morphology, respectively. The results show that highly dispersed PtCo nanoalloys with average sizes of ca. 2.4 nm are dispersed on the surfaces of RGO sheets. The catalytic performances of the PtCo/RGO, Pt/RGO, and Pt/C catalysts toward methanol oxidation were studied, which indicates that PtCo/RGO nanoalloys show higher catalytic performance and stability than Pt/RGO and Pt/C catalysts. Furthermore, the PtCo/RGO catalyst exhibits improved CO-tolerance ability due to the addition of Co.







Similar content being viewed by others
References
Tian J, Sun G, Jiang L, Yan S, Mao Q, Xin Q (2007) Highly stable PtRuTiOx/C anode electrocatalyst for direct methanol fuel cells. Electrochem Commun 9:563–568
Antolini E, Salgado JRC, Gonzalez ER (2005) Carbon supported Pt75M25 (M = Co, Ni) alloys as anode and cathode electrocatalysts for direct methanol fuel cells. J Electroanal Chem 580:145–154
Kang Y, Murray CB (2010) Synthesis and electrocatalytic properties of cubic Mn − Pt nanocrystals (nanocubes). J Am Chem Soc 132:7568–7569
Huang H, Sun D, Wang X (2012) PtCo alloy nanoparticles supported on graphene nanosheets with high performance for methanol oxidation. Chin Sci Bull 57:3071–3079
Cao J, Guo M, Wu J, Xu J, Wang W, Chen Z (2015) Carbon-supported Ag@Pt core–shell nanoparticles with enhanced electrochemical activity for methanol oxidation and oxygen reduction reaction. J Power Sources 277:155–160
Tao L, Dou S, Ma Z, Wang S (2015) Platinum nanoparticles supported on nitrobenzene-functionalized multiwalled carbon nanotube as efficient electrocatalysts for methanol oxidation reaction. Electrochim Acta 157:46–53
Huang H, Chen H, Sun D, Wang X (2012) Graphene nanoplate-Pt composite as a high performance electrocatalyst for direct methanol fuel cells. J Power Sources 204:46–52
Gupta VK, Atar N, Yola ML, Üstündağ Z, Uzun L (2014) A novel magnetic Fe@Au core–shell nanoparticles anchored graphene oxide recyclable nanocatalyst for the reduction of nitrophenol compounds. Water Res 48:210–217
Yola ML, Gupta VK, Eren T, Şen AE, Atar N (2014) A novel electro analytical nanosensor based on graphene oxide/silver nanoparticles for simultaneous determination of quercetin and morin. Electrochim Acta 120:204–211
Yola ML, Atar N, Üstündağ Z, Solak AO (2013) A novel voltammetric sensor based on p-aminothiophenol functionalized graphene oxide/gold nanoparticles for determining quercetin in the presence of ascorbic acid. J Electroanal Chem 698:9–16
Atar N, Eren T, Yola ML (2015) Ultrahigh capacity anode material for lithium ion battery based on rod gold nanoparticles decorated reduced graphene oxide. Thin Solid Films 590:156–162
Gao H, Liao S, Zeng J, Xie Y, Dang D (2011) Preparation and characterization of core–shell structured catalysts using PtxPdy as active shell and nano-sized Ru as core for potential direct formic acid fuel cell application. Electrochim Acta 56:2024–2030
Xue Q, Yang ZY (2016) Remarkable enhancement of the specific activities of Pt catalysts to methanol oxidation through tuning the surface properties of supporting materials by fast microwave treatments. Int J Hydrogen Energy 41:6310–6315
Duan J, Zhang X, Yuan W, Chen H, Jiang S, Liu X, Zhang Y, Chang L, Sun Z, Du J (2015) Graphene oxide aerogel-supported Pt electrocatalysts for methanol oxidation. J Power Sources 285:76–79
Ma JH, Wang L, Mu X, Li L (2015) Nitrogen-doped graphene supported Pt nanoparticles with enhanced performance for methanol oxidation. Int J Hydrog Energy 40:2641–2647
Atar N, Eren T, Yola ML, Karimi-Maleh H, Demirdogen B (2015) Magnetic iron oxide and iron oxide@gold nanoparticle anchored nitrogen and sulfur-functionalized reduced graphene oxide electrocatalyst for methanol oxidation. RSC Adv 5:26402–26409
Atar N, Eren T, Demirdögen B, Yola ML, Çağlayan MO (2015) Silver, gold, and silver@gold nanoparticle-anchored l-cysteine-functionalized reduced graphene oxide as electrocatalyst for methanol oxidation. Ionics 21:2285–2293
Gupta VK, Yola ML, Atar N, Üstündağ Z, Solak AO (2014) Electrochemical studies on graphene oxide-supported metallic and bimetallic nanoparticles for fuel cell applications. J Mol Liq 191:172–176
Hummers WS, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80:1339–1339
Maillard F, Lu GQ, Wieckowski A, Stimming U (2005) Ru-decorated Pt surfaces as model fuel cell electrocatalysts for CO electrooxidation. J Phys Chem B 109:16230–16243
Xu Y, Bai H, Lu G, Li C, Shi G (2008) Flexible graphene films via the filtration of water-soluble noncovalent functionalized graphene sheets. J Am Chem Soc 130:5856–5857
Ji Z, Shen X, Zhu G, Chen K, Fu G, Tong L (2012) Enhanced electrocatalytic performance of Pt-based nanoparticles on reduced graphene oxide for methanol oxidation. J Electroanal Chem 682:95–100
Xiong L, Kannan AM, Manthiram A (2002) Pt–M (M = Fe, Co, Ni and Cu) electrocatalysts synthesized by an aqueous route for proton exchange membrane fuel cells. Electrochem Commun 4:898–903
Xing Y (2004) Synthesis and electrochemical characterization of uniformly-dispersed high loading Pt nanoparticles on sonochemically-treated carbon nanotubes. J Phys Chem B 108:19255–19259
Hu Y, Wu P, Zhang H, Cai C (2012) Synthesis of graphene-supported hollow Pt-Ni nanocatalysts for highly active electrocatalysis toward the methanol oxidation reaction. Electrochim Acta 85:314–321
Feng JX, Zhang QL, Wang AJ, Wei J, Chen JR, Feng JJ (2014) Caffeine-assisted facile synthesis of platinum@palladium core-shell nanoparticles supported on reduced graphene oxide with enhanced electrocatalytic activity for methanol oxidation. Electrochim Acta 142:343–350
Mukerjee S, Srinivasan S, Soriaga MP, McBreen J (1995) Role of structural and electronic properties of Pt and Pt alloys on electrocatalysis of oxygen reduction: an in situ XANES and EXAFS investigation. J Electrochem Soc 142:1409–1422
Zhong RS, Qin YH, Niu DF, Zhang XS, Zhou XG, Sun SG, Yuan WK (2013) Effect of carbon nanofiber surface groups on oxygen reduction reaction of supported Pt electrocatalyst. Electrochim Acta 89:157–162
Jiang R, Rong C, Chu D (2011) Surface coverage of Pt atoms on PtCo nanoparticles and catalytic kinetics for oxygen reduction. Electrochim Acta 56:2532–2540
Pozio A, De Francesco M, Cemmi A, Cardellini F, Giorgi L (2002) Comparison of high surface Pt/C catalysts by cyclic voltammetry. J Power Sources 105:13–19
Chetty R, Xia W, Kundu S, Bron M, Reinecke T, Schuhmann W, Muhler M (2009) Effect of reduction temperature on the preparation and characterization of Pt − Ru nanoparticles on multiwalled carbon nanotubes. Langmuir 25:3853–3860
Li Y, Gao W, Ci L, Wang C, Ajayan PM (2010) Catalytic performance of Pt nanoparticles on reduced graphene oxide for methanol electro-oxidation. Carbon 48:1124–1130
Acknowledgments
This work was supported by the National Natural Science Foundation of China (no. U1404201), Foundation for Young Core Teacher by Zhengzhou University of Light Industry (no. 2013XGGJS007), Graduate’s Scientific Research Foundation of Zhengzhou University of Light Industry (no. 2015002), and Program for Technology Innovation Team in Universities of Henan Province, China (16IRTSTHN016).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gao, H., He, L., Xiao, Y. et al. One-step synthesis of reduced graphene oxide-supported PtCo nanoalloys with enhanced electrocatalytic activity for methanol oxidation. Ionics 22, 2175–2182 (2016). https://doi.org/10.1007/s11581-016-1727-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-016-1727-9