Abstract
Background
Individual contributions of exogenous Schwann cells (SCs) and vascular endothelial growth factor (VEGF) were evaluated in acellular nerve allografts (ANAs). ANA processing removes SCs and vasculature, likely contributing to reduced regeneration compared to autografts. Exogenous SCs may improve the regenerative microenvironment, and VEGF has been shown to stimulate angiogenesis. Replacing these components in ANAs may improve regeneration.
Methods
A rat sciatic nerve transection model was used to study 20-mm grafts. Four graft types were studied: (1) isograft, (2) ANA, (3) ANA-SCs, and (4) ANA-VEGF. After 10 weeks in vivo, the midgraft and distal nerve to the grafts were analyzed for axonal regeneration using histomorphometry to assess total myelinated axon counts, density, width, and percent neural tissue.
Results
The most axons in the distal nerve were regenerated in the isograft followed by the ANA- SC group, with 9171 ± 1822 and 7103 ± 1576 regenerated axons respectively. Both the ANA and ANA-VEGF groups had significantly fewer regenerated axons compared to the isograft (p < 0.05) with 5225 ± 2994 and 5709 ± 2657 regenerated axons, respectively. The ANA and ANA-VEGF groups also had significantly reduced fiber density and percent nerve compared to the isograft; the isograft and ANA-SC groups were not significantly different (p < 0.05).
Conclusions
These results show that SCs improve axonal regeneration in a 20 mm ANA to a greater extent than VEGF. VEGF treatment showed a trend toward increased axonal regeneration but was not significantly different compared to the untreated ANA. The role of VEGF may be clearer in longer grafts where ischemia is a greater factor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nerve regeneration across processed or acellular nerve allografts (ANAs) represents an important goal in therapeutics for nerve injury. ANAs have several advantages over nerve autografts and synthetic conduits. Autografting requires donor nerves, with associated morbidity and increased operating time [32, 38]. In contrast, ANAs are readily available “off the shelf” in a variety of sizes. Synthetic conduits have similar ease of availability and lack of donor site morbidity; however, they lack the endoneurial microstructure found in autografts and ANAs. As a result, ANAs have superior efficacy versus conduits in short gap nerve repairs [25, 42].
While ANAs demonstrate potential as a grafting material for short nerve defects, comparison to autografts finds ANAs lacking and deters their use in more challenging reconstructions, such as long gap, large diameter nerve injuries. In animal studies, myelinated axon counts in short (10–20 mm) ANAs are less than 50 % of those in isografts at early, more sensitive, time points [21, 34, 42]; as ANA length increases, this regeneration worsens [34, 39]. Functional outcome measures have varied considerably. Some studies show comparable outcomes in muscle mass and walking track assessment when comparing isografts and ANAs, while others show isograft superiority [21, 34, 40, 42]. In the largest clinical trial of ANAs, 87 % of patients had meaningful recovery, defined as S3-4 and M3-M5, following repair with ANAs in short nerve gaps averaging 22 mm. However, only 18 % of the sensory nerve repairs returned S4 recovery and similarly, only 19 % of motor nerve injuries had M5 recovery [7, 10]. These outcomes are comparable to those achieved clinically with autografts in short nerve gaps, but still far from normal function [5, 13, 45]. By improving regeneration in ANAs, achieving greater functional recovery may be possible, and longer ANAs could be utilized for more challenging reconstructions, where the need is greatest.
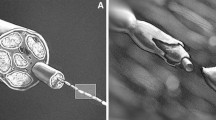
Decellularizing allografts to create ANAs is critical to preventing rejection [19]. However, the loss of Schwann cells (SCs) and blood vessels containing endothelial cells likely contributes significantly to the reduced efficacy of ANAs compared to isografts (Fig. 1a, b). Adding exogenous SCs, the cellular drivers of regeneration, is a possible means for improvement. ANAs rely on host SCs to populate the graft and to recreate the regenerative microenvironment [14, 15, 41]. Because axonal regeneration follows SC migration, any delay in the migration of host SCs delays regeneration [16]. A similar issue exists for vascularization; autografts are able to revascularize more rapidly than ANAs due to inosculation of the preexisting endothelial cell-lined vascular network [3, 4]. A promising stimulus for angiogenesis in nerve grafts is vascular endothelial growth factor (VEGF), which has been shown to accelerate angiogenesis in multiple models [9, 27, 28].
Processing of an ANA removes cells, most significantly the Schwann cells (SCs) that direct nerve regeneration and endothelial cells that line the vasculature (b). Iso(auto)grafts retain those cells and, as a result, revascularize more rapidly through a process of inosculation and better support nerve regeneration compared to ANAs (a). In this study, we examine replacing what is lost: adding exogenous SCs (c) or adding a growth factor, VEGF, to promote angiogenesis (d)
The objective of this study was to assess whether the addition of exogenous SCs and VEGF improves axonal regeneration in ANAs in comparison to isografts. We studied two experimental groups in a 20 mm ANA model. SCs were added to ANAs to provide support via local neurotrophic factors to regenerating axons (ANA-SC), and VEGF was added to ANAs to promote angiogenesis (ANA-VEGF; Fig. 1c, d, respectively). We hypothesize that both of these treatments will increase axonal regeneration through the ANAs so as to be more comparable to isografts.
Materials and Methods
Animal Surgeries
Rats were randomized to one of the following groups: (1) isograft (Iso), (2) ANA, (3) ANA-SCs, and (4) ANA-VEGF. For each of the four groups, n = 8 animals underwent sciatic nerve surgery. All institutional and national guidelines for the care and use of laboratory animals were followed. Adult male Lewis rats (200–250 g, Charles River Laboratories, Wilmington, MA) were anesthetized with ketamine (75 mg/kg, Fort Dodge Animal Health, Fort Dodge, IA) and dexmedetomidine (0.5 mg/kg, Pfizer Animal Health, Exton, PA). Surgeries were done with aseptic technique using an operating microscope (JEDMED/KAPS, St. Louis, MO). The right sciatic nerve was exposed and transected 5 mm proximal to the distal trifurcation. A 20 mm graft was sutured in place using 9–0 nylon suture at each end. The repair was tension-free, and a two-layer closure of muscle and skin was performed. Animals were recovered and housed in a central animal care facility and provided with food (PicoLab rodent diet 20, Purina Mills Nutrition International, St. Louis, MO) and water ad libitum. All animals were monitored postoperatively for infection and distress. After 10 weeks, the ANAs and a 1 cm portion of the distal nerve were excised and collected. Rats were euthanized with intraperitoneal injections of Somnasol (150 mg/kg, Delmarva Laboratories, Des Moines, IA).
SC Culture
SCs were cultured as previously described [6, 31]. Briefly, SCs were harvested from the sciatic nerve of Lewis rats and incubated in growth medium composed of Dulbecco’s modified Eagle medium (DMEM, Invitrogen) supplemented with 10 % heat-inactivated fetal bovine serum (Sigma-Aldrich) and 100 U/mL penicillin, 100 μg/mL streptomycin, 0.25 μg/mL amphotericin B, 20 μg/mL bovine pituitary extract (Biomedical Tech, Inc., Stoughton, MA), and 5 μM forskolin (Sigma-Aldrich) for 7 days. The nerves were then treated with 0.5 % collagenase IV (Worthington, Lakewood, NJ) and 1.25 U/mL dispase (Worthington) in growth medium at 37 °C for 30 min. The digest was then strained and centrifuged at 400×g for 6 min to collect the cell component. Cells were cultured on poly-l-lysine (Sigma-Aldrich)-coated tissue culture dishes (BD Falcon, Bedford, MA). To isolate the SCs after 6 days in culture, fibroblasts were complement killed through treatment with anti-Thy 1.1 antibody (1:40 dilution in media, Serotec, Raleigh, NC) and rabbit complement (1:4 dilution in media, Sigma-Aldrich).
ANA Preparation
ANAs were decellularized from the sciatic nerves of Sprague-Dawley rats using a previously published detergent processing protocol [18, 19]. In brief, the nerves were repeatedly washed in deionized water and three detergents in a sodium-phosphate buffer: Triton X-200, sulfobetaine-16, and sulfobetaine-10. These washes are completed over 4 days, and the ANAs stored at 4 °C until use. The VEGF-treated ANAs were prepared using a fibrin matrix as previously described by Sakiyama-Elbert et al. [35]. ANAs were cut to 30 mm and clamped at one end with a 1 mm microvascular clamp to prevent leakage of injected solutions. The fibrin matrix was prepared by mixing the following components (final concentrations provided): human plasminogen-free fibrinogen containing Factor XIII (4.0 mg/mL, EMD Millipore, Billerica, MA), bovine thrombin (2 NIH U/mL, Sigma-Aldrich), CaCl2 (2.5 mM, Sigma-Aldrich), and recombinant human VEGF (0.83 μg/mL, Peprotech). The fibrinogen was kept separate from the other components until just prior to injection into the ANA; all solutions and Hamilton™ syringes (Hamilton Company, Reno, NV) were kept on ice to prevent premature polymerization. The fibrin matrix solution was injected slowly in a sub-epineurial plane of the ANA with a 27G Hamilton™ syringe, and any solution that leaked was removed using a pipette to avoid polymerization on the exterior. This procedure was repeated at eight injection sites for a final gel volume of 20 μL per 10 mm of graft. The concentration of VEGF in the ANA was 3 ng/mm of graft for a total dose of 60 ng. Injected ANAs were allowed to polymerize at 37 °C for 1 h prior to surgery. For the SC-treated group, cultured SCs were treated with 0.25 % trypsin and centrifuged at 130×g for 5 min and resuspended in growth media at a concentration of 106 cells/8 μL. The cell suspension was injected along the length of the graft in a sub-epineurial plane using a 27G Hamilton™ syringe. A total of 106 cells were injected into each graft [21, 22]. SC-supplemented grafts were implanted immediately following injection.
Histomorphometry
En bloc specimens of the graft and sciatic nerve distal to the implanted grafts underwent histomorphometric analysis as previously described [20]. Briefly, the nerves are preserved in 3 % glutaraldehyde (Polysciences Inc., Warrington, PA), fixed in 1 % osmium tetroxide, and serially dehydrated in ethanol. The nerves were then embedded in epoxy (Polysciences) and sectioned on an ultramicrotome into 1 μm cross sections. Slides were stained with 1 % toluidine blue dye. The slides were then analyzed at 1000× on a Leitz Laborlux S microscope. The Leco IA32 Image Analysis System (Leco, St. Joseph, MI) was utilized to quantify nerve fiber counts, fiber width, fiber density, and percent neural tissue. All analysis was done by an observer blinded to the experimental groups.
Statistical Analysis
All data were compiled as mean ± standard deviation, and a one-factor ANOVA was used to examine means from the histomorphometry data. If analysis showed a significant difference, a Newman Keul’s post hoc was performed. A significance level of p < 0.05 was used in all statistical tests performed.
Results
Nerve Graft Harvest
The effect of VEGF and exogenous SC transplantation into 20 mm ANAs was evaluated in vivo in a rat sciatic nerve transection model. All grafts were successfully explanted after 10 weeks. The grafts showed successful integration at both the proximal and distal nerve stumps.
Histology
All grafts resulted in myelinated fibers in the distal nerve demonstrating successful axonal growth through the graft (Fig. 2). The architecture of the nerve appears more organized with more uniform arrangement and size of fibers in the isograft, ANA-VEGF, and ANA-SC groups compared to the unmodified ANA group. Nerve fiber density is also qualitatively greater in the ANA-SC and ANA-VEGF groups compared to the ANA group and more similar to the isografts. The ANA qualitatively appears to have more non-neuronal tissue and greater variation in the fiber size. Normal blood vessels are visible in sections from all of the groups.
Representative histological images of the distal nerve demonstrating successful axonal growth through the nerve grafts after 10 weeks in vivo. Sections show healthy myelinated fibers with mature architecture in all groups. The Iso and ANA-SC group show greater fiber density and number compared to the untreated ANA and ANA-VEGF group. Scale bar = 50 μm
Histomorphometry
To assess the quantity and characteristics of the regenerated axons, nerve cross sections were taken at the midgraft and 3–5 mm from the distal coaptation of the grafts and evaluated with morphometric quantification.
At the midgraft, the isograft had the most fibers, followed by the ANA-SC, the ANA-VEGF, and the ANA: respectively, 15,593 ± 2551, 8460 ± 2104, 7534 ± 2385, and 6815 ± 2104. All group fiber numbers were statistically decreased compared to the isograft (p < 0.05). In the distal nerve, the isograft regenerated the largest number of myelinated axons (9171 ± 1822), while the untreated ANAs regenerated the fewest (5225 ± 2994, Fig. 3a). The VEGF and SC treatments produced 5709 ± 2657 and 7103 ± 1576 regenerated axons, respectively. Both the ANA and ANA-VEGF groups were statistically different compared to the isograft (p < 0.05), while the ANA-SC group was statistically similar to the isograft (p > 0.05). A normal rat sciatic nerve contains ~8000 nerve fibers [43].
To evaluate the quality and maturity of nerve regeneration, the same cross sections were quantified for nerve fiber density, percent neural tissue, and fiber width. At the midgraft, percent nerve was highest in the isograft (33.0 ± 5.5 %) and was significantly different compared to the ANA (23.3 ± 5.9 %). The ANA-SC and ANA-VEGF were higher compared to the ANA, but not significantly different (25.9 ± 6.4 and 28.5 ± 5.6 %, respectively). Density was similar across all groups at the midgraft, ranging 27,698–34,478 fibers/mm3. Fiber width ranged 2.8–2.9 μm and was not significantly different across the groups.
In the distal nerve, as with total myelinated axon number, the ANA and ANA-VEGF group had reduced percent nerve and nerve fiber density compared to the isograft (Fig. 3b, c), while the ANA-SC group was not significantly different from the isograft. Fiber width was used to evaluate the maturity of the fibers (Fig. 3d). All of the groups showed similar widths in the range of 2.3–3.2 μm. Normal rat sciatic nerve has a nerve fiber density of 11,882 fibers/mm3 and an average fiber width of 6.5 μm [29].
Discussion
ANAs are effective scaffolds for SC migration and axonal regeneration in short gap, small diameter nerve injuries [10, 30]. However, as the grafts increase in length, there is increasing SC replicative burden and a greater temporal delay in repopulating the graft, both of which likely contribute to reduced regeneration [16, 34, 39]. Moreover, recent data suggests that replicative burden and stress may contribute to SC senescence, further reducing the regenerative capacity of ANAs [34]. Cell replacement and growth factor delivery strategies can be employed to improve these grafts toward the goal of full recovery and utilization in long gap models. In this study, the strategy was to add components that are lost in ANA processing: SCs and VEGF. By adding factors previously shown to improve axonal regeneration and angiogenesis in separate treatment studies, we sought to indirectly determine which component plays a more critical role in successful axonal regeneration.
We elucidate that SCs are pivotal to recreating a regenerative microenvironment: SC addition resulted in grafts with similar regeneration to isografts in terms of number of regenerated fibers, nerve fiber density, and percent neural tissue. Comparing to the midgraft, the presence of SCs appears to have facilitated more fibers regenerating to the distal nerve. The work presented here is consistent with prior work adding SCs to ANAs. Jesuraj et al. showed a significant increase in regenerated nerve fibers in a 14 mm ANA supplemented with 1 × 106 SCs [21]. However, the benefit of SC addition was not as dramatic in this study; we show a 35 % increase, while Jesuraj et al. demonstrated a near doubling of fiber regeneration compared to the ANAs alone. A key difference is that the ANAs used by Jesuraj et al. were generated by the cold preservation method. Moore et al. showed that the ANA processing method used in this study, detergent processing, far outperformed cold preservation for nerve fiber regeneration, resulting in 84 % nerve fiber regeneration compared to isograft as opposed to 25 % in cold preserved grafts [21, 30]. The difference in benefits seen by SC addition may thus be attributed to an improved baseline graft in our studies with the detergent-processed ANA. While the addition of SCs ultimately improves regeneration in both types of ANAs, in contrast, Fox et al. showed that there was no benefit to the addition of SCs to a 15 mm ANA [12]. However, their ANAs were supplemented with 1/10th the number of cells used in this study, which likely contributes to the difference. These alternative studies illustrate that while SCs can be a beneficial additive to ANAs, both the recipient scaffold and cell quantity play a role in their efficacy. The ANA processing technique and SC quantity used in our study were chosen based on previous work demonstrating they best facilitate nerve regeneration [21, 22, 30].
VEGF is a compelling additive to ANAs because it may enhance axonal regeneration indirectly through angiogenesis and through direct action on axonal growth. Vascularization is a key difference between ANAs and autografts; autografts revascularize through inosculation within 72 h of implantation via the existing endothelial cell-lined vasculature [3, 4], while ANAs depend on the more lengthy process of angiogenesis. Reducing the graft ischemic time may facilitate regeneration. In vitro, exogenous VEGF interacts with receptors on SCs and regenerating axons to promote neuronal survival, SC proliferation, and axonal outgrowth [1, 8, 23, 24, 36]. Sondell et al. showed that VEGF treatment of ANAs resulted in increased graft vascularization and changes to SC morphology and receptor expression. However, the time course for that study was 10 days, too early to observe an effect on axonal growth [37]. Rovak et al. studied VEGF treatment in 20 mm ANAs kept in vivo for 15 weeks in a rat sciatic nerve transection model but showed conflicting results: VEGF treatment resulted in increased axon regeneration in the proximal graft, but the no difference in distal nerve axon counts [33]. Rovak et al. may have seen improvements at the proximal nerve as a result of axonal sprouting [44].
Dosing and delivery of VEGF is a complex part of the puzzle and may explain why significant effects on axonal regeneration were not observed. In the prior work combining VEGF and ANAs, the ANA was soaked in solutions of varying VEGF concentration [33, 37]. In conduits, VEGF has been suspended in and released from Matrigel or PLGA microspheres [17, 26]. In contrast, we use a fibrin suspension of VEGF at a dose of 3 ng/mm of graft; this dose had previously been found to promote earlier angiogenesis in ANAs compared to untreated ANAs (unpublished data). This dose is higher than that used by Hobson et al., wherein they also demonstrated increased vasculature [17]. Further studies to elucidate dosages that optimize the separate vasculogenesis and axonal regenerative actions of VEGF are warranted.
In conclusion, this study demonstrates that exogenous SCs improved axonal regeneration through ANAs, while VEGF did not result in increased regeneration as hypothesized. These additives may be more powerful in longer grafts than the short model studied here. Longer grafts have a longer ischemic time (unpublished data) and greater SC replicative burden, contributing to chronic cellular stress, which can result in cellular senescence [2, 11]. Previous studies demonstrated substantial axonal regeneration up to and into the distal nerve in 20 mm ANAs. However, 40 mm ANAs demonstrated axonal regeneration to the middle of the graft (~20 mm of growth), while 60 mm ANAs only regenerated ~10 mm into the graft closer examination of 60 mm ANAs that showed accumulation of senescent SCs [34]. Senescent SC accumulation is hypothesized to play a role in the disproportionately reduced regeneration associated with increasing ANA length. Future work will focus on using SCs and VEGF in longer ANAs to reduce the accumulation of senescent SCs and, thus, improve axonal regeneration.
References
Bearden SE, Segal SS. Microvessels promote motor nerve survival and regeneration through local VEGF release following ectopic reattachment. Microcirculation. 2004;11:633–44.
Ben-Porath I, Weinberg RA. The signals and pathways activating cellular senescence. Int J Biochem Cell Biol. 2005;37:961–76.
Best TJ, Mackinnon SE, Evans PJ, et al. Peripheral nerve revascularization: histomorphometric study of small- and large-caliber grafts. J Reconstr Microsurg. 1999;15:183–90.
Best TJ, Mackinnon SE, Midha R, et al. Revascularization of peripheral nerve autografts and allografts. Plast Reconstr Surg. 1999;104:152–60.
Birch R, Raji AR. Repair of median and ulnar nerves. Primary suture is best. J Bone Joint Surg (Br). 1991;73:154–7.
Brockes JP, Raff MC. Studies on cultured rat Schwann cells. II. Comparison with a rat Schwann cell line. In Vitro. 1979;15:772–8.
Brooks DN, Weber RV, Chao JD, et al. Processed nerve allografts for peripheral nerve reconstruction: a multicenter study of utilization and outcomes in sensory, mixed, and motor nerve reconstructions. Microsurgery. 2012;32:1–14.
Brushart TM. Chapter 9: nerve regeneration. Nerve repair: the scientific basis. Cary: Oxford University Press; 2011. p. 250–352.
Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307.
Cho MS, Rinker BD, Weber RV, et al. Functional outcome following nerve repair in the upper extremity using processed nerve allograft. J Hand Surg [Am]. 2012;37:2340–9.
Dimri GP, Campisi J. Molecular and cell biology of replicative senescence. Cold Spring Harb Symp Quant Biol. 1994;59:67–73.
Fox IK, Schwetye KE, Keune JD, et al. Schwann-cell injection of cold-preserved nerve allografts. Microsurgery. 2005;25:502–7.
Frykman G, Gramyk K. Results of nerve grafting. In: Gelberman R, editor. Operative nerve repair and reconstruction. Philadelphia: JB Lippincott; 1991.
Hall SM. The effect of inhibiting Schwann cell mitosis on the re-innervation of acellular autografts in the peripheral nervous system of the mouse. Neuropathol Appl Neurobiol. 1986;12:401–14.
Hall SM. Regeneration in cellular and acellular autografts in the peripheral nervous system. Neuropathol Appl Neurobiol. 1986;12:27–46.
Hayashi A, Koob JW, Liu DZ, et al. A double-transgenic mouse used to track migrating Schwann cells and regenerating axons following engraftment of injured nerves. Exp Neurol. 2007;207:128–38.
Hobson MI, Green CJ, Terenghi G. VEGF enhances intraneural angiogenesis and improves nerve regeneration after axotomy. J Anat. 2000;197(Pt 4):591–605.
Hudson TW, Liu SY, Schmidt CE. Engineering an improved acellular nerve graft via optimized chemical processing. Tissue Eng. 2004;10:1346–58.
Hudson TW, Zawko S, Deister C, et al. Optimized acellular nerve graft is immunologically tolerated and supports regeneration. Tissue Eng. 2004;10:1641–51.
Hunter DA, Moradzadeh A, Whitlock EL, et al. Binary imaging analysis for comprehensive quantitative histomorphometry of peripheral nerve. J Neurosci Methods. 2007;166:116–24.
Jesuraj NJ, Santosa KB, Macewan MR, et al. Schwann cells seeded in acellular nerve grafts improve functional recovery. Muscle Nerve. 2013.
Jesuraj NJ, Santosa KB, Newton P, et al. A systematic evaluation of Schwann cell injection into acellular cold-preserved nerve grafts. J Neurosci Methods. 2011;197:209–15.
Jin K, Mao XO, Greenberg DA. Vascular endothelial growth factor stimulates neurite outgrowth from cerebral cortical neurons via Rho kinase signaling. J Neurobiol. 2006;66:236–42.
Jin K, Zhu Y, Sun Y, et al. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2002;99:11946–50.
Karabekmez FE, Duymaz A, Moran SL. Early clinical outcomes with the use of decellularized nerve allograft for repair of sensory defects within the hand. Hand (N Y). 2009;4:245–9.
Karagoz H, Ulkur E, Kerimoglu O, et al. Vascular endothelial growth factor-loaded poly(lactic-co-glycolic acid) microspheres-induced lateral axonal sprouting into the vein graft bridging two healthy nerves: nerve graft prefabrication using controlled release system. Microsurgery. 2012;32:635–41.
Laschke MW, Rucker M, Jensen G, et al. Incorporation of growth factor containing Matrigel promotes vascularization of porous PLGA scaffolds. J Biomed Mater Res A. 2008;85:397–407.
Lindhorst D, Tavassol F, von See C, et al. Effects of VEGF loading on scaffold-confined vascularization. J Biomed Mater Res A. 2010;95:783–92.
Mackinnon SE, Dellon AL, O’Brien JP. Changes in nerve fiber numbers distal to a nerve repair in the rat sciatic nerve model. Muscle Nerve. 1991;14:1116–22.
Moore AM, MacEwan M, Santosa KB, et al. Acellular nerve allografts in peripheral nerve regeneration: a comparative study. Muscle Nerve. 2011;44:221–34.
Raff MC, Abney E, Brockes JP, et al. Schwann cell growth factors. Cell. 1978;15:813–22.
Rajput K, Reddy S, Shankar H. Painful neuromas. Clin J Pain. 2012;28:639–45.
Rovak JM, Mungara AK, Aydin MA, et al. Effects of vascular endothelial growth factor on nerve regeneration in acellular nerve grafts. J Reconstr Microsurg. 2004;20:53–8.
Saheb-Al-Zamani M, Yan Y, Farber SJ, et al. Limited regeneration in long acellular nerve allografts is associated with increased Schwann cell senescence. Exp Neurol. 2013;247C:165–77.
Sakiyama-Elbert SE, Hubbell JA. Controlled release of nerve growth factor from a heparin-containing fibrin-based cell ingrowth matrix. J Control Release. 2000;69:149–58.
Sondell M, Lundborg G, Kanje M. Vascular endothelial growth factor has neurotrophic activity and stimulates axonal outgrowth, enhancing cell survival and Schwann cell proliferation in the peripheral nervous system. J Neurosci. 1999;19:5731–40.
Sondell M, Lundborg G, Kanje M. Vascular endothelial growth factor stimulates Schwann cell invasion and neovascularization of acellular nerve grafts. Brain Res. 1999;846:219–28.
Staniforth P, Fisher TR. The effects of sural nerve excision in autogenous nerve grafting. Hand. 1978;10:187–90.
Strasberg SR, Mackinnon SE, Genden EM, et al. Long-segment nerve allograft regeneration in the sheep model: experimental study and review of the literature. J Reconstr Microsurg. 1996;12:529–37.
Tang P, Kilic A, Konopka G, et al. Histologic and functional outcomes of nerve defects treated with acellular allograft versus cabled autograft in a rat model. Microsurgery. 2013;33:460–7.
Whitlock EL, Myckatyn TM, Tong AY, et al. Dynamic quantification of host Schwann cell migration into peripheral nerve allografts. Exp Neurol. 2010;225:310–9.
Whitlock EL, Tuffaha SH, Luciano JP, et al. Processed allografts and type I collagen conduits for repair of peripheral nerve gaps. Muscle Nerve. 2009;39:787–99.
Wood MD, Kemp SW, Liu EH, et al. Rat-derived processed nerve allografts support more axon regeneration in rat than human-derived processed nerve xenografts. J Biomed Mater Res A. 2014;102:1085–91.
Wood MD, Kemp SW, Weber C, et al. Outcome measures of peripheral nerve regeneration. Ann Anat. 2011;193:321–33.
Yang M, Rawson JL, Zhang EW, et al. Comparisons of outcomes from repair of median nerve and ulnar nerve defect with nerve graft and tubulization: a meta-analysis. J Reconstr Microsurg. 2011;27:451–60.
Funding
This work was supported in part by grants from the National Institutes of Neurological Disorders and Stroke of the National Institutes of Health (R01 NS051706 and R56 NS33406), the Plastic Surgery Foundation, and the American Society for Peripheral Nerve.
Conflict of Interest
The authors declare that they have no conflict of interest.
Statement of Human and Animal Rights
All institutional and national guidelines for the care and use of laboratory animals were followed.
Statement of Informed Consent
This study does not contain any studies with human subjects.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Hoben, G., Yan, Y., Iyer, N. et al. Comparison of acellular nerve allograft modification with Schwann cells or VEGF. HAND 10, 396–402 (2015). https://doi.org/10.1007/s11552-014-9720-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11552-014-9720-0