Abstract
Purpose
Progression of hip osteoarthritis (hip OA) leads to pain and disability, likely leading to surgical treatment such as hip arthroplasty at the terminal stage. The severity of hip OA is often classified using the Crowe and Kellgren–Lawrence (KL) classifications. However, as the classification is subjective, we aimed to develop an automated approach to classify the disease severity based on the two grades using digitally-reconstructed radiographs from CT images.
Methods
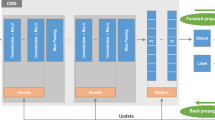
Automatic grading of the hip OA severity was performed using deep learning-based models. The models were trained to predict the disease grade using two grading schemes, i.e., predicting the Crowe and KL grades separately, and predicting a new ordinal label combining both grades and representing the disease progression of hip OA. The models were trained in classification and regression settings. In addition, the model uncertainty was estimated and validated as a predictor of classification accuracy. The models were trained and validated on a database of 197 hip OA patients, and externally validated on 52 patients. The model accuracy was evaluated using exact class accuracy (ECA), one-neighbor class accuracy (ONCA), and balanced accuracy.
Results
The deep learning models produced a comparable accuracy of approximately 0.65 (ECA) and 0.95 (ONCA) in the classification and regression settings. The model uncertainty was significantly larger in cases with large classification errors (\(P< 6\text {e}{-}3\)).
Conclusions
In this study, an automatic approach for grading hip OA severity from CT images was developed. The models have shown comparable performance with high ONCA, which facilitates automated grading in large-scale CT databases and indicates the potential for further disease progression analysis. Classification accuracy was correlated with the model uncertainty, which would allow for the prediction of classification errors. The code will be made publicly available at https://github.com/NAIST-ICB/HipOA-Grading.









Similar content being viewed by others
References
Hoy DG, Smith E, Cross M, Sanchez-Riera L, Buchbinder R, Blyth FM, Brooks P, Woolf AD, Osborne RH, Fransen M, Driscoll T, Vos T, Blore JD, Murray C, Johns N, Naghavi M, Carnahan E, March LM (2014) The global burden of musculoskeletal conditions for 2010: an overview of methods. Ann Rheum Dis 73(6):982–989. https://doi.org/10.1136/annrheumdis-2013-204344
Günther KP, Sun Y (1999) Reliability of radiographic assessment in hip and knee osteoarthritis. Osteoarthr Cartil 7(2):239–246. https://doi.org/10.1053/joca.1998.0152
Damen J, Schiphof D, Wolde ST, Cats HA, Bierma-Zeinstra SMA, Oei EHG (2014) Inter-observer reliability for radiographic assessment of early osteoarthritis features: the check (cohort hip and cohort knee) study. Osteoarthr Cartil 22(7):969–974. https://doi.org/10.1016/j.joca.2014.05.007
Üreten K, Arslan T, Gültekin KE, Demir AND, Özer HF, Bilgili Y (2020) Detection of hip osteoarthritis by using plain pelvic radiographs with deep learning methods. Skelet Radiol 49:1369–1374. https://doi.org/10.1007/s00256-020-03433-9
von Schacky CE, Sohn JH, Liu F, Ozhinsky E, Jungmann PM, Nardo L, Posadzy M, Foreman SC, Nevitt MC, Link TM, Pedoia V (2020) Development and validation of a multitask deep learning model for severity grading of hip osteoarthritis features on radiographs. Radiology 295(1):136–145. https://doi.org/10.1148/radiol.2020190925
Turmezei TD, Fotiadou A, Lomas DJ, Hopper MA, Poole KES (2014) A new CT grading system for hip osteoarthritis. Osteoarthr Cartil 22(10):1360–1366. https://doi.org/10.1016/j.joca.2014.03.008
Gebre RK, Hirvasniemi J, van der Heijden RA, Lantto I, Saarakkala S, Leppilahti J, Jämsä T (2022) Detecting hip osteoarthritis on clinical CT: a deep learning application based on 2-D summation images derived from CT. Osteoporos Int 33(2):355–365. https://doi.org/10.1007/s00198-021-06130-y
He K, Zhang X, Ren S, Sun J (2016) Deep residual learning for image recognition. In: Proceedings of the IEEE conference on computer vision and pattern recognition, pp 770–778. https://doi.org/10.1109/CVPR.2016.90
Simonyan K, Zisserman A (2014) Very deep convolutional networks for large-scale image recognition. arXiv preprint arXiv:1409.1556, https://doi.org/10.48550/arXiv.1409.1556
Huang G, Liu Z, Van Der Maaten L, Weinberger KQ (2017) Densely connected convolutional networks. In: Proceedings of the IEEE conference on computer vision and pattern recognition, pp 4700–4708. https://doi.org/10.1109/CVPR.2017.243
Joseph GB, McCulloch CE, Nevitt MC, Link TM, Sohn JH (2022) Machine learning to predict incident radiographic knee osteoarthritis over 8 years using combined MR imaging features, demographics, and clinical factors: data from the osteoarthritis initiative. Osteoarthr Cartil 30(2):270–279. https://doi.org/10.1016/j.joca.2021.11.007
Guan B, Liu F, Mizaian AH, Demehri S, Samsonov A, Guermazi A, Kijowski R (2022) Deep learning approach to predict pain progression in knee osteoarthritis. Skelet Radiol. https://doi.org/10.1007/s00256-021-03773-0
He K, Gan C, Li Z, Rekik I, Yin Z, Ji W, Gao Y, Wang Q, Zhang J, Shen D (2023) Transformers in medical image analysis. Intell Med 3(1):59–78. https://doi.org/10.1016/j.imed.2022.07.002
Dosovitskiy A, Beyer L, Kolesnikov A, Weissenborn D, Zhai X, Unterthiner T, Dehghani M, Minderer M, Heigold G, Gelly S, Uszkoreit J, Houlsby N (2021) An image is worth \(16\times 16\) words: transformers for image recognition at scale. ICLR. https://doi.org/10.48550/arXiv.2010.11929
Konwer A, Xu X, Bae J, Chen C, Prasanna P (2022) Temporal context matters: enhancing single image prediction with disease progression representations. In: Proceedings of the IEEE/CVF conference on computer vision and pattern recognition (CVPR), pp 18824–18835. https://doi.org/10.1109/CVPR52688.2022.01826
Vaswani A, Shazeer N, Parmar N, Uszkoreit J, Jones L, Gomez AN, Kaiser Ł, Polosukhin I (2017) Attention is all you need. In: Proceedings of the 31st international conference on neural information processing systems, NIPS’17, pp 6000–6010. https://doi.org/10.48550/arXiv.1706.03762
Hiasa Y, Otake Y, Takao M, Ogawa T, Sugano N, Sato Y (2019) Automated muscle segmentation from clinical CT using Bayesian U-Net for personalized musculoskeletal modeling. IEEE Trans Med Imaging 39(4):1030–1040. https://doi.org/10.1109/tmi.2019.2940555
Roy AG, Navab N, Wachinger C (2018) Concurrent spatial and channel ‘squeeze & excitation’ in fully convolutional networks. In: Medical image computing and computer assisted intervention—MICCAI 2018: 21st international conference, Granada, Spain, September 16–20, 2018, Proceedings, Part I, pp 421–429. https://doi.org/10.1007/978-3-030-00928-1_48
Çiçek Ö, Abdulkadir A, Lienkamp SS, Brox T, Ronneberger O (2016) 3D U-Net: learning dense volumetric segmentation from sparse annotation. In: Medical image computing and computer-assisted intervention—MICCAI 2016: 19th international conference, Athens, Greece, October 17–21, Proceedings, Part II 19. Springer, pp 424–432
Uemura K, Otake Y, Takashima K, Hamada H, Imagama T, Takao M, Sakai T, Sato Y, Okada S, Sugano N (2023) Development and validation of an open-source tool for opportunistic screening of osteoporosis from hip CT images. Bone 0115:R1. https://doi.org/10.1302/2046-3758.129.BJR-2023-0115.R1
Gal Y, Ghahramani Z (2016) Dropout as a Bayesian approximation: representing model uncertainty in deep learning. In: International conference on machine learning, pp 1050–1059. https://doi.org/10.48550/arXiv.1506.02142
Inoue K, Wicart P, Kawasaki T, Huang J, Ushiyama T, Hukuda S, Courpied J-P (2000) Prevalence of hip osteoarthritis and acetabular dysplasia in French and Japanese adults. Rheumatology 39(7):745–748. https://doi.org/10.1093/rheumatology/39.7.745
Hadley NA, Brown TD, Weinstein SL (1990) The effects of contact pressure elevations and aseptic necrosis on the long-term outcome of congenital hip dislocation. J Orthop Res 8(4):504–513. https://doi.org/10.1002/jor.1100080406
Deng J, Dong W, Socher R, Li L-J, Li K, Li F-F (2009) ImageNet: a large-scale hierarchical image database. In: 2009 IEEE conference on computer vision and pattern recognition, pp 248–255. https://doi.org/10.1109/CVPR.2009.5206848
Lin T-Y, Goyal P, Girshick R, He K, Dollar P (2017) Focal loss for dense object detection. In: Proceedings of the IEEE international conference on computer vision (ICCV). https://doi.org/10.1109/ICCV.2017.324
Kingma DP, Ba J (2014) Adam: a method for stochastic optimization. arXiv preprint arXiv:1412.6980, https://doi.org/10.48550/arXiv.1412.6980
Buslaev A, Iglovikov VI, Khvedchenya E, Parinov A, Druzhinin M, Kalinin AA (2020) Albumentations: fast and flexible image augmentations. Information. https://doi.org/10.3390/info11020125
Paszke A, Gross S, Chintala S, Chanan G, Yang E, DeVito Z, Lin Z, Desmaison A, Antiga L, Lerer A (2017) Automatic differentiation in Pytorch. In: NIPS-W. https://github.com/pytorch/pytorch
TorchVision maintainers and contributors. Torchvision: Pytorch’s computer vision library, 2016. https://github.com/pytorch/vision
Wan K, Yang S, Feng B, Ding Y, Xie L (2019) Reconciling feature-reuse and overfitting in DenseNet with specialized dropout. In: 2019 IEEE 31st international conference on tools with artificial intelligence (ICTAI), pp 760–767. IEEE. https://doi.org/10.1109/ICTAI.2019.00110
McInnes L, Healy J, James M (2018) UMAP: uniform manifold approximation and projection for dimension reduction
Acknowledgements
This work was funded by MEXT/JSPS KAKENHI (19H01176, 20H04550, 21K16655, 21K18080).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Nothing to declare.
Ethics approval
Ethical approval was obtained from the Institutional Review Boards (IRBs) of the institutions participating in this study (IRB approval numbers: 21115 for Osaka University Hospital and 2020-M-7 for Nara Institute of Science and Technology.)
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Masuda, M., Soufi, M., Otake, Y. et al. Automatic hip osteoarthritis grading with uncertainty estimation from computed tomography using digitally-reconstructed radiographs. Int J CARS (2024). https://doi.org/10.1007/s11548-024-03087-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11548-024-03087-1