Abstract
Background
Pazopanib is the only tyrosine kinase inhibitor approved for the treatment of patients with advanced soft tissue sarcoma (STS) who have received prior chemotherapy, but there have been limited real-world data on pazopanib for the treatment of advanced STS.
Objective
We aimed to evaluate clinical outcomes of pazopanib in patients with multiple histologic STS types in real-world settings.
Patients and Methods
We retrospectively analyzed clinical data of Korean patients with advanced STS treated with pazopanib between 2008 and 2019. Outcomes of interest included treatment response, survival according to histologic subtypes, and adverse events.
Results
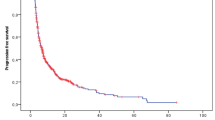
The analysis included 347 STS patients. The disease control rate for all pazopanib-treated patients was 54.8% (95% confidence interval (CI) 49.5–60.0); 54 patients (15.6%) achieved a partial response and 136 (39.2%) had stable disease. Patients with alveolar soft-part sarcoma (ASPS; 90%), solitary fibrous tumor (SFT; 88.2%), synovial sarcoma (66.7%), leiomyosarcoma (61.1%), and undifferentiated pleomorphic sarcoma (59.6%) showed higher disease control rates than those with other STS subtypes. Overall, median progression-free survival (PFS) and overall survival (OS) were 5.3 months (95% CI 4.5–6.0) and 12 months (95% CI 10–14), respectively. Noticeable survival outcomes occurred in patients with ASPS and SFT, with a median PFS of 24.5 (95% CI 2.5–30.0) and 13.0 (95% CI 3.0–21.3) months, respectively. The median OS of patients with ASPS and SFT was 48 (95% CI 17–52) and 32 (95% CI 19–66) months, respectively. Adverse drug reactions occurred in 170 patients (49.0%) but were not life-threatening.
Conclusions
This real-world data analysis showed acceptable efficacy and tolerability of pazopanib in patients pretreated with cytotoxic chemotherapy for advanced STS, with favorable treatment outcomes for ASPS and SFT.




Similar content being viewed by others

References
Skubitz KM, D’Adamo DR. Sarcoma. Mayo Clin Proc. 2007;82(11):1409–32.
Clark MA, Fisher C, Judson I, Thomas JM. Soft-tissue sarcomas in adults. N Engl J Med. 2005;353(7):701–11.
Kim HS, Nam CM, Jang SY, Choi SK, Han M, Kim S, et al. Characteristics and treatment patterns of patients with advanced soft tissue sarcoma in Korea. Cancer Res Treat. 2019;51(4):1380–91.
Sleijfer S, Ray-Coquard I, Papai Z, Le Cesne A, Scurr M, Schöffski P, et al. Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: a phase II study from the European Organisation for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group (EORTC study 62043). J Clin Oncol. 2009;27(19):3126–32.
Lee DY, Staddon AP, Shabason JE, Sebro R. Phase I and phase II clinical trials in sarcoma: Implications for drug discovery and development. Cancer Med. 2019;8(2):585–92.
Sleijfer S, Quali M, van Glabbeke M, Krarup-Hansen A, Rodenhuis S, Le Cesne A, et al. Prognostic and predictive factors for outcome to first-line ifosfamide-containing chemotherapy for adult patients with advanced soft tissue sarcomas: an exploratory, retrospective analysis on large series from the European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group (EORTC-STBSG). Eur J Cancer. 2010;46(1):72–83.
van der Graaf WT, Blay JY, Chawla SP, Kim DW, Bui-Nguyen B, Casali PG, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012;379(9829):1879–86.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 11). Eur J Cancer. 2009;45(2):228–47.
Yoo KH, Kim HS, Lee SJ, Park SH, Kim SJ, Kim SH, et al. Efficacy of pazopanib monotherapy in patients who had been heavily pretreated for metastatic soft tissue sarcoma: a retrospective case series. BMC Cancer. 2015;15:154.
Nakamura T, Matsumine A, Kawai A, Araki N, Goto T, Yonemoto T, et al. The clinical outcome of pazopanib treatment in Japanese patients with relapsed soft tissue sarcoma: a Japanese Musculoskeletal Oncology Group (JMOG) study. Cancer. 2016;122(9):1408–16.
Gelderblom H, Judson IR, Benson C, Merimsky O, Grignani G, Katz D, et al. Treatment patterns and clinical outcomes with pazopanib in patients with advanced soft tissue sarcomas in a compassionate use setting: results of the SPIRE study. Acta Oncol. 2017;56(12):1769–75.
Kim JH, Park HS, Heo SJ, Kim SK, Han JW, Shin KH, et al. Differences in the efficacies of pazopanib and gemcitabine/docetaxel as second-line treatments for metastatic soft tissue sarcoma. Oncology. 2019;96(2):59–69.
Jaber OI, Kirby PA. Alveolar soft part sarcoma. Arch Pathol Lab Med. 2015;139(11):1459–62.
Kummar S, Allen D, Monks A, Polley EC, Hose CD, Ivy SP, et al. Cediranib for metastatic alveolar soft part sarcoma. J Clin Oncol. 2013;31(18):2296–302.
Stacchiotti S, Negri T, Zaffaroni N, Palassini E, Morosi C, Brich S, et al. Sunitinib in advanced alveolar soft part sarcoma: evidence of a direct antitumor effect. Ann Oncol. 2011;22(7):1682–90.
Stacchiotti S, Mir O, Le Cesne A, Vincenzi B, Fedenko A, Maki RG, et al. Activity of pazopanib and trabectedin in advanced alveolar soft part sarcoma. Oncologist. 2018;23(1):62–70.
Kim M, Kim TM, Keam B, Kim YJ, Paeng JC, Moon KC, et al. A phase II trial of pazopanib in patients with metastatic alveolar soft part sarcoma. Oncologist. 2019;24(1):20–e29.
Judson I, Morden JP, Kilburn L, Leahy M, Benson C, Bhadri V, et al. Cediranib in patients with alveolar soft-part sarcoma (CASPS): double-blind, placebo-controlled, randomised, phase 2 trial. Lancet Oncol. 2019;20(7):1023–34.
Paoluzzi L, Maki RG. Diagnosis, prognosis, and treatment of alveolar soft-part sarcoma: a review. JAMA Oncol. 2019;5(2):254–60.
Stockwin LH, Vistica DT, Kenney S, Schrump DS, Butcher DO, Raffeld M, et al. Gene expression profiling of alveolar soft-part sarcoma (ASPS). BMC Cancer. 2009;9:22.
Goodwin ML, Jin H, Straessler K, Smith-Fry K, Zhu JF, Monument MJ, et al. Modeling alveolar soft part sarcomagenesis in the mouse: a role for lactate in the tumor microenvironment. Cancer Cell. 2014;26(6):851–62.
Park MS, Araujo DM. New insights into the hemangiopericytoma/solitary fibrous tumor spectrum of tumors. Curr Opin Oncol. 2009;21(4):327–31.
Levard A, Derbel O, Méeus P, Ranchère D, Ray-Coquard I, Blay JY, et al. Outcome of patients with advanced solitary fibrous tumors: the Centre Léon Bérard experience. BMC Cancer. 2013;13:109.
Park MS, Ravi V, Conley A, Patel SR, Trent JC, Lev DC, et al. The role of chemotherapy in advanced solitary fibrous tumors: a retrospective analysis. Clin Sarcoma Res. 2013;3(1):7.
Stacchiotti S, Negri T, Libertini M, Palassini E, Marrari A, De Troia B, et al. Sunitinib malate in solitary fibrous tumor (SFT). Ann Oncol. 2012;23(12):3171–9.
Maruzzo M, Martin-Liberal J, Messiou C, Miah A, Thway K, Alvarado R, et al. Pazopanib as first line treatment for solitary fibrous tumours: the Royal Marsden Hospital experience. Clin Sarcoma Res. 2015;5:5.
Park MS, Patel SR, Ludwig JA, Trent JC, Conrad CA, Lazar AJ, et al. Activity of temozolomide and bevacizumab in the treatment of locally advanced, recurrent, and metastatic hemangiopericytoma and malignant solitary fibrous tumor. Cancer. 2011;117(21):4939–47.
Martin-Broto J, Stacchiotti S, Lopez-Pousa A, Redondo A, Bernabeu D, de Alava E, et al. Pazopanib for treatment of advanced malignant and dedifferentiated solitary fibrous tumour: a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2019;20(1):134–44.
Schöffski P, Cornillie J, Wozniak A, Li H, Hompes D. Soft tissue sarcoma: an update on systemic treatment options for patients with advanced disease. Oncol Res Treat. 2014;37(6):355–62.
Author information
Authors and Affiliations
Contributions
Study concept: Jeong Eun Kim and Tae Won Kim. Study design: Jeong Eun Kim. Data acquisition: Jeong Eun Kim, Jung Yong Hong, Jee Hung Kim, Hyo Song Kim, and Jin-Hee Ahn. Quality control of the data and algorithms: Jeong Eun Kim. Data analysis and interpretation: Jeong Eun Kim, Ji Sung Lee, and Chung Ryul Oh. Statistical analysis: Ji Sung Lee. Article preparation: Chung Ryul Oh and Jeong Eun Kim. Article editing: Chung Ryul Oh. Article review: Jeong Eun Kim, Jung Yong Hong, Jee Hung Kim, and Hyo Song Kim.
Corresponding author
Ethics declarations
Funding
This work was funded by a 2017 cancer research support project from the Korea Foundation for Cancer Research (CB-2017-B-2), and was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant number HI18C2383).
Conflict of interest
Chung Ryul Oh, Jung Yong Hong, Jee Hung Kim, Ji Sung Lee, Hyo Song Kim, Tae Won Kim, Jin-Hee Ahn, and Jeong Eun Kim declare they have no conflicts of interest that might be relevant to the contents of this manuscript.
Consent for publication
The authors declare that the manuscript has not been published previously, in whole or in part, and is not under consideration for publication elsewhere. All authors have approved the manuscript and consent to its publication.
Ethics approval
This study was approved by the Asan Medical Center Institutional Review Board (IRB No. 2017-1098).
Availability of data and material
The data contain potentially identifiable patient information and cannot be shared publicly due to ethical and legal restrictions.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Oh, C.R., Hong, J.Y., Kim, J.H. et al. Real-World Outcomes of Pazopanib Treatment in Korean Patients with Advanced Soft Tissue Sarcoma: A Multicenter Retrospective Cohort Study. Targ Oncol 15, 485–493 (2020). https://doi.org/10.1007/s11523-020-00731-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-020-00731-z