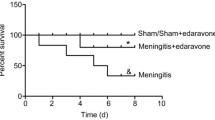
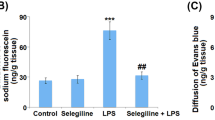
Abstract
Bacterial meningitis (BM) is the main cause of the central nervous system (CNS) infection and continues to be an important cause of mortality and morbidity. Glutathione (GSH), an endogenous tripeptide antioxidant, has been proved to exert crucial role in reducing superoxide radicals, hydroxyl radicals and peroxynitrites. The purpose of this study is to expand the application scope of GSH via exploring its therapeutic effect on BM caused by Salmonella typhimurium SL1344 and then provide a novel approach for the treatment of BM. The results suggested that intragastric administration of GSH could significantly increase median survival and improve experimental autoimmune encephalomyelitis score of BM model mice. However, exogenous GSH did not affect the adhesion, invasion and cytotoxicity of SL1344 to C6, BV2 and primary microglia. Due to the contradiction between the therapeutic and bactericidal effects of GSH, the effect of GSH on blood-brain barrier (BBB) was investigated to explore its action target for the treatment of meningitis. GSH was found to repair the damage of BBB and then prevent the leakage of SL1344 from the brain to the blood circulation. The repaired BBB could also effectively reduce the entry of macrophages and neutrophils into the brain, and significantly reverse the microglia activation induced by SL1344. More importantly, exogenous GSH was proved to reduce mouse brain cell apoptosis by inhibiting the activation of caspase-8 followed by caspase-3, and reversing the up-regulation of ICAD and PARP-1 caused by SL1344.
Graphical Abstract








Similar content being viewed by others
References
Bhan MK, Bahl R, Bhatnagar S (2005) Typhoid and paratyphoid fever. In: Lancet
Block ML, Zecca L, Hong JS (2007) Microglia-mediated neurotoxicity: uncovering the molecular mechanisms.Nat. Rev. Neurosci.8
Brouwer MC, Tunkel AR, Van De Beek D (2010) Epidemiology, diagnosis, and antimicrobial treatment of acute bacterial meningitis.Clin. Microbiol. Rev.23
Cazanave S, Berson A, Haouzi D et al (2007) High hepatic glutathione stores alleviate Fas-induced apoptosis in mice. J Hepatol 46. https://doi.org/10.1016/j.jhep.2006.11.015
Chang CC, Omarjee S, Lim A et al (2013) Chemokine levels and chemokine receptor expression in the blood and the cerebrospinal fluid of HIV-infected patientswith cryptococcal meningitis and cryptococcosis- Associated immune reconstitution inflammatory syndrome. J Infect Dis 208. https://doi.org/10.1093/infdis/jit388
Chen C, Ding Q, Shen B et al (2020) Insights into the authentic active ingredients and action sites of oral exogenous glutathione in the treatment of ischemic brain injury based on pharmacokinetic-pharmacodynamic studies S. Drug Metab Dispos 48. https://doi.org/10.1124/DMD.119.089458
Collins SM, Bercik P (2009) The relationship between intestinal microbiota and the Central Nervous System in normal gastrointestinal function and disease. https://doi.org/10.1053/j.gastro.2009.01.075. Gastroenterology 136:
Coureuil M, Lécuyer H, Bourdoulous S, Nassif X (2017) A journey into the brain: insight into how bacterial pathogens cross blood-brain barriers.Nat. Rev. Microbiol.15
Doran KS, Fulde M, Gratz N et al (2016) Host–pathogen interactions in bacterial meningitis.Acta Neuropathol.131
Drevets DA, Leenen PJM, Greenfield RA (2004) Invasion of the central nervous system by intracellular Bacteria.Clin. Microbiol. Rev.17
Forman HJ, Zhang H, Rinna A (2009) Glutathione: overview of its protective roles, measurement, and biosynthesis.Mol. Aspects Med.30
Franco R, Cidlowski JA (2009) Apoptosis and glutathione: beyond an antioxidant.Cell Death Differ.16
Friesen C, Kiess Y, Debatin KM (2004) A critical role of glutathione in determining apoptosis sensitivity and resistance in leukemia cells. Cell Death Differ 11. https://doi.org/10.1038/sj.cdd.4401431
Hanigan MH, Ricketts WA (1993) Extracellular glutathione is a source of cysteine for cells that express gamma-glutamyl transpeptidase. Biochemistry 32:6302–6306
Haraga A, Ohlson MB, Miller SI (2008) Salmonellae interplay with host cells.Nat. Rev. Microbiol.6
Helms M, Vastrup P, Gerner-Smidt P, Mølbak K (2002) Excess mortality associated with antimicrobial drug-resistant Salmonella typhimurium. Emerg Infect Dis 8. https://doi.org/10.3201/eid0805.010267
Hickman S, Izzy S, Sen P et al (2018) Microglia in neurodegeneration.Nat. Neurosci.21
Huang JC, Yue ZP, Yu HF et al (2022) TAZ ameliorates the microglia-mediated inflammatory response via the Nrf2-ROS-NF-κB pathway. Mol Ther - Nucleic Acids. https://doi.org/10.1016/j.omtn.2022.03.025. 28:
Hussain T, Tan B, Yin Y et al (2016) Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016
Ifuku M, Hinkelmann L, Kuhrt LD et al (2020) Activation of toll-like receptor 5 in microglia modulates their function and triggers neuronal injury. Acta Neuropathol Commun 8. https://doi.org/10.1186/s40478-020-01031-3
Islam MT (2017) Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders.Neurol. Res.39
Kim KS (2006) Microbial translocation of the blood-brain barrier.Int. J. Parasitol.36
Kim SY, Buckwalter M, Soreq H et al (2012) Blood-brain barrier dysfunction-induced inflammatory signaling in brain pathology and epileptogenesis.Epilepsia53 Suppl 6
Klein M, Koedel U, Pfister HW (2006) Oxidative stress in pneumococcal meningitis: A future target for adjunctive therapy? Prog. Neurobiol. 80
Knuff-Janzen K, Tupin A, Yurist-Doutsch S et al (2020) Multiple Salmonella-pathogenicity island 2 effectors are required to facilitate bacterial establishment of its intracellular niche and virulence. PLoS ONE 15. https://doi.org/10.1371/journal.pone.0235020
Koedel U, Frankenberg T, Kirschnek S et al (2009) Apoptosis is essential for neutrophil functional shutdown and determines tissue damage in experimental pneumococcal meningitis. PLoS Pathog 5. https://doi.org/10.1371/journal.ppat.1000461
Koelman DLH, Brouwer MC, Van De Beek D (2019) Targeting the complement system in bacterial meningitis. Brain 142. https://doi.org/10.1093/brain/awz222
Kreutzberg GW (1996) Microglia: A sensor for pathological events in the CNS. Trends Neurosci. 19
Kumar DKV, Choi HS, Washicosky KJ et al (2016) Amyloid-β peptide protects against microbial infection in mouse and worm models of Alzheimer’s disease. Sci Transl Med 8. https://doi.org/10.1126/scitranslmed.aaf1059
Majeed S, Radotra BD, Sharma S (2016) Adjunctive role of MMP-9 inhibition along with conventional anti-tubercular drugs against experimental tuberculous meningitis. Int J Exp Pathol 97. https://doi.org/10.1111/iep.12191
Manoharan S, Guillemin GJ, Abiramasundari RS et al (2016) The Role of Reactive Oxygen Species in the Pathogenesis of Alzheimer’s Disease, Parkinson’s Disease, and Huntington’s Disease: A Mini Review. Oxid. Med. Cell. Longev. 2016
Neher JJ, Neniskyte U, Zhao J-W et al (2011) Inhibition of Microglial phagocytosis is sufficient to prevent inflammatory neuronal death. J Immunol 186. https://doi.org/10.4049/jimmunol.1003600
Peppas S, Pansieri C, Piovani D et al (2021) The brain-gut axis: psychological functioning and inflammatory bowel diseases.J. Clin. Med.10
Prager O, Friedman A, Nebenzahl YM (2017) Role of neural barriers in the pathogenesis and outcome of Streptococcus pneumoniae meningitis (review).Exp. Ther. Med.13
Prinz M, Jung S, Priller J (2019) Microglia Biology: One Century of Evolving Concepts. Cell 179
Ramakrishnan M, Ulland AJ, Steinhardt LC et al (2009) Sequelae due to bacterial meningitis among african children: a systematic literature review. BMC Med 7. https://doi.org/10.1186/1741-7015-7-47
Ramesh G, Maclean AG, Philipp MT (2013) Cytokines and chemokines at the crossroads of neuroinflammation, neurodegeneration, and neuropathic pain. https://doi.org/10.1155/2013/480739. Mediators Inflamm 2013:
Salimi H, Klein RS (2019) Disruption of the blood-brain barrier during Neuroinflammatory and Neuroinfectious Diseases. In: Neuroimmune Diseases
Soeters HM, Diallo AO, Bicaba BW et al (2019) Bacterial meningitis epidemiology in five countries in the Meningitis Belt of Sub-Saharan Africa, 2015–2017. J. Infect. Dis. 220
Swann O, Everett DB, Furyk JS et al (2014) Bacterial meningitis in malawian infants < 2 months of age: etiology and susceptibility to World Health Organization first-line antibiotics. Pediatr Infect Dis J 33. https://doi.org/10.1097/INF.0000000000000210
Szabó C (2003) Multiple pathways of peroxynitrite cytotoxicity. In: Toxicology Letters
Tramutola A, Lanzillotta C, Perluigi M, Butterfield DA (2017) Oxidative stress, protein modification and Alzheimer disease.Brain Res. Bull.133
Wang H, Du Y, sha, Xu W, shuo et al (2021) Exogenous glutathione exerts a therapeutic effect in ischemic stroke rats by interacting with intrastriatal dopamine. Acta Pharmacol Sin. https://doi.org/10.1038/s41401-021-00650-3
Wickham ME, Brown NF, Provias J et al (2007) Oral infection of mice with Salmonella enterica serovar typhimurium causes meningitis and infection of the brain. BMC Infect Dis 7. https://doi.org/10.1186/1471-2334-7-65
Yang I, Han SJ, Kaur G et al (2010) The role of microglia in central nervous system immunity and glioma immunology.J. Clin. Neurosci.17
Yang L, Jiménez JA, Earley AM et al (2020) Drainage of inflammatory macromolecules from brain to periphery targets the liver for macrophage infiltration. Elife 9. https://doi.org/10.7554/eLife.58191
Yong HH, Suhn HK, Sung ZK, Woo HP (2008) Apoptosis in arsenic trioxide-treated Calu-6 lung cells is correlated with the depletion of GSH levels rather than the changes of ROS levels. J Cell Biochem 104. https://doi.org/10.1002/jcb.21673
Zhang D, Xu S, Wang Y, Zhu G (2021) The potentials of melatonin in the prevention and treatment of bacterial meningitis disease. Molecules 26. https://doi.org/10.3390/molecules26051419
Zhu N, Zhang C, Prakash A et al (2021) Therapeutic development of group B Streptococcus meningitis by targeting a host cell signaling network involving EGFR. EMBO Mol Med 13. https://doi.org/10.15252/emmm.202012651
Funding
This work was supported by the National Natural Science Foundation of China (Grant No.: 82274194); the Jiangsu Natural Science Funds (Grant No.: BK20211224); the Project of State Key Laboratory of Natural Medicines, China Pharmaceutical University (Grant No.: SKLNMZZ202001) and the Natural Science Foundation of Hebei Province (Grant No.: H2020208022, H2020208025, H2021302001, C2021418001, H2021208006).
Author information
Authors and Affiliations
Contributions
This study was conceived and designed by LY, WGJ and CYG. GHM and JW were responsible for data curation, analysis and original draft writing. LSJ and XL provided the comprehensive technical support. MSY, LKAQ and ZZH contributed to the inspection of data and final manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that there are no conflicts of interest.
Compliance with Ethical Standards
This research was approved by the Animal Ethics Committee of China Pharmaceutical University.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Huimin Guo, Wei Jin, and Keanqi Liu these authors contributed equally to this work.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, H., Jin, W., Liu, K. et al. Oral GSH Exerts a Therapeutic Effect on Experimental Salmonella Meningitis by Protecting BBB Integrity and Inhibiting Salmonella-induced Apoptosis. J Neuroimmune Pharmacol 18, 112–126 (2023). https://doi.org/10.1007/s11481-022-10055-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-022-10055-6