Abstract
Background
The ideal local anesthetic for use in ambulatory spinal anesthesia is safe, with minimal adverse effects, and of a duration that does not impede post-anesthesia care unit (PACU) discharge. Since its approval for use in spinal anesthesia in Europe in 2012, chloroprocaine has seen a resurgence. Recent studies have investigated the safety and efficacy of preservative-free chloroprocaine for use in spinal anesthesia, but few provide the incidence of adverse events such as urinary retention and transient neurologic symptoms.
Questions/Purposes
We sought to assess the safety of chloroprocaine for spinal anesthesia, including the incidence of adverse events and the duration and quality of its use, in the initial 6 months of its use at our institution. We hypothesized that chloroprocaine would provide effective spinal anesthesia for orthopedic cases of short duration, with a low rate of complications.
Methods
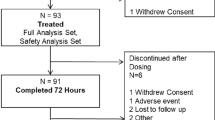
We conducted a retrospective chart review of all patients from June to December 2016 at our institution who had ambulatory knee arthroscopy or foot procedures in which chloroprocaine spinal anesthesia was used. For all 445 charts analyzed, data were collected on anesthesia characteristics, office visits, nursing PACU assessment, and nursing post-operative day 1 follow-up phone calls.
Results
The median chloroprocaine dosage was 44 mg (interquartile range [IQR], 40 to 50). The median duration of sensory block was 156 min (IQR, 128 to 189) and of motor block was 148 min (IQR, 123 to 181). Time to ambulation was 186 min (IQR, 158 to 218) and time to meeting of discharge criteria was 218 min (IQR, 189 to 250). The most common adverse events in the PACU were bradycardia and hypotension. No patients had urinary retention or transient neurologic symptoms.
Conclusions
In 6 months of use at our institution, chloroprocaine provided safe and effective spinal anesthesia for short orthopedic procedures, with no incidence of transient neurologic symptoms, neuropraxia, or urinary retention.

Similar content being viewed by others
References
Camponovo, C. Spinal 1% 2-Chloroprocaine versus general anesthesia for ultra-short outpatient procedures: a retrospective analysis. Acta Biomed. 2014;85(3):265–278.
Casati A, Danelli G, Berti M, et al. Intrathecal 2-chloroprocaine for lower limb outpatient surgery: a prospective, randomized, double-blind, clinical evaluation. Anesth Analg. 2006;103(1):234–238.
Forster J, Rosenberg PH. Revival of old local anesthetics for spinal anesthesia in ambulatory surgery. Curr Opinion Anesthesiol. 2011;24:633–637.
Gebhart V, Hausen S, Weiss C, Schmittner MD. Using chloroprocaine for spinal anaesthesia in outpatient knee-arthroscopy results in earlier discharge and improved operating room efficiency compared to mepivacaine and prilocaine. Knee Surg Sports Traumatol Arthrosc. 2018. doi: https://doi.org/10.1007/s00167-018-5327-2.
Ghisi D, Bonarelli S. Ambulatory surgery with chloroprocaine spinal anesthesia: a review. Ambulatory Anesthesia. 2015; 2:111–120.
Goldblum E, Atchabahian A. The use of 2-chloroprocaine for spinal anesthesia. Acta Anaesthesiologica Scandinavica. 2013;57:545–552.
Hejtmanek MR, Pollock JE. Chloroprocaine for spinal anesthesia: a retrospective analysis. Acta Anaesthesiol Scand. 2011;55(3):267–72.
Keld DB, Hein L, Dalgaard M, Krogh L, Rodt SÅ. The incidence of transient neurologic symptoms (TNS) after spinal anaesthesia in patients undergoing surgery in the supine position. Hyperbaric lidocaine 5% versus hyperbaric bupivacaine 0.5%. Acta Anaesthesiol Scand. 2000;44:285–290.
Kouri M, Kopacz DJ. Spinal 2-chloroprocaine: a comparison with lidocaine in volunteers. Anesth Analg. 2004; 98(1):75–80.
Lacasse MA, Roy JD, Forget J, Vandenbroucke F, Seal RF, Beauliu D, McCormack M, Massicotte L. Comparison of bupivacaine and 2-chloroprocaine for spinal anesthesia for outpatient surgery: a double-blind randomized trial. Can J Anesth. 2011;58:384–391.
Mulroy MF. Outpatients do not need to void after short neuraxial bocks. Anesthesiology. 2009;111(6):1388.
Mulroy MF, Salinas FV, Larkin KL, Polissar NL. Ambulatory surgery patients may be discharged before voiding after short-acting spinal and epidural anesthesia. Anesthesiology. 2002;97:315–319.
Saporito A, Ceppi M, Perren A, et. al. Does spinal chloroprocaine pharmacokinetic profile actually translate into clinical advantage in terms of clinical outcomes when compared to low-dose spinal bupivacaine? A systematic review and meta-analysis. J Clin Anesth. 2019;52:99–104.
Sell A, Tein T, Pitkanen M. Spinal 2-chloroprocaine: effective dose for ambulatory surgery. Acta Anaesthesiol Scand. 2008;52(5):695–699.
Teunkens A, Vermeulen K, Van Gerven E, Fieuws S, Van de Velde M, Rex S. Comparison of 2-chloroprocaine, bupivacaine, and lidocaine for spinal anesthesia in patients undergoing knee arthroscopy in an outpatient setting: a double-blind randomized controlled trial. Reg Anesth Pain Med. 2016;41(5):576–583.
YaDeau JT, Liguori GA, Zayas VM. The incidence of transient neurologic symptoms after spinal anesthesia with mepivacaine. Anesth Analg. 2005;101(3):661–675.
Yoos JR, Kapacz DJ. Spinal 2-chloroprocaine for surgery: an initial 10-month experience. Anesth Analg. 2005;100(2):553–558.
Funding
This study was funded by the Department of Anesthesiology, Critical Care & Pain Management, Hospital for Special Surgery.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
David H. Kim, MD; Richard Kahn, MD; Andrew Lee, MD; Phuong Dinh Mac, BS; Yu-fen Chiu, PhD; Jacques Yadeau, MD, PhD; and Jiabin Liu, MD, PhD declare that they have no conflicts of interest.
Human/Animal Rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2013.
Informed Consent
Informed consent was waived from all patients for being included in this study.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Additional information
Level of Evidence: Level IV: Therapeutic Study
Rights and permissions
About this article
Cite this article
Kim, D.H., Kahn, R., Lee, A. et al. Chloroprocaine Provides Safe, Effective, Short-Acting Spinal Anesthesia Ideal for Ambulatory Surgeries: A Retrospective Review. HSS Jrnl 16 (Suppl 2), 280–284 (2020). https://doi.org/10.1007/s11420-019-09713-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11420-019-09713-y