Abstract
Purpose
A sensitive method was developed for the simultaneous determination of midazolam and propofol in human plasma samples via modified quick, easy, cheap, effective, rugged, and safe (QuEChERS) extraction for their analysis by gas chromatography–tandem mass spectrometry (GC–MS/MS).
Methods
Detailed optimization and validation experiments were conducted; plasma quality control samples spiked with two drugs and two internal standards (ISs) were extracted using the QuEChERS technique and determined by GC–MS/MS. The present method was applied to the measurements in an actual clinical case.
Results
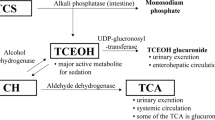
The regression equations for midazolam and propofol in plasma displayed good linearity (r > 0.9978) from 10 to 1000 ng/mL with a limit of quantification of 10 ng/mL. The intra- and interday precisions for both drugs were not greater than 8.6%. The accuracies of quantitation ranged from 98 to 110%. The extraction efficiencies of midazolam and propofol for plasma were 93 to 117%. The method was successfully applied to the analyses of plasma midazolam and propofol concentrations in a brain-injured patient subjected to hypothermia, which validated the present methodology.
Conclusions
In this study, the authors have successfully established the detailed procedure for GC–MS/MS analysis of midazolam and propofol in human plasma. The sensitivity and specificity of the present method for the drugs were almost comparable to those by the standard liquid chromatography–tandem mass spectrometry. To their knowledge, this is the first report for analysis of midazolam/propofol by GC–MS/MS.



Similar content being viewed by others
References
Mihic SJ, Mayfield J, Harris RA (2017) Hypnotics and sedatives. In: Brunton LL, Hilal-Dandan R, Knollmann BC (eds) Goodman & Gilman's the pharmacological basis of therapeutics, 13th edn. McGraw-Hill, New York, pp 339–354
Zaporowska-Stachowiak I, Szymański K, Oduah M-T, Stachowiak-Szymczak K, Łuczak J, Sopata M (2019) Midazolam: safety of use in palliative care: a systematic critical review. Biomed Pharmacother 114:108838. https://doi.org/10.1016/j.biopha.2019.108838(open access article)
Rey A, Rossetti A, Miroz J-P, Eckert P, Oddo M (2019) Late awakening in survivors of postanoxic coma: early neurophysiologic predictors and association with ICU and long-term neurologic recovery. Crit Care Med 47:85–92. https://doi.org/10.1097/CCM.0000000000003470
Bjelland TW, Klepstad P, Haugen BO, Nilsen T, Dale O (2013) Effects of hypothermia on the disposition of morphine, midazolam, fentanyl, and propofol in intensive care unit patients. Drug Metab Dispos 41:214–223. https://doi.org/10.1124/dmd.112.045567
Bjelland TW, Dale O, Kaisen K, Haugen BO, Lydersen S, Strand K, Klepstad P (2012) Propofol and remifentanil versus midazolam and fentanyl for sedation during therapeutic hypothermia after cardiac arrest: a randomised trial. Intensive Care Med 38:958–967. https://doi.org/10.1007/s00134-012-2540-1
Fukuoka N, Aibiki M, Tsukamoto T, Seki K, Morita S (2004) Biphasic concentration change during continuous midazolam administration in brain-injured patients undergoing therapeutic moderate hypothermia. Resuscitation 60:225–230. https://doi.org/10.1016/j.resuscitation2003.09.017
Arayakarnkul P, Chomtho K (2019) Treatment options in pediatric super-refractory status epilepticus. Brain Dev 41:359–366. https://doi.org/10.1016/j.braindev.2018.11.011
Yeary J, Garavaglia J, McKnight R, Smith M (2018) Change in management of status epilepticus with the addition of neurointensivist-led neurocritical care team at a rural academic medical center. Hosp Pharm 53:303–307. https://doi.org/10.1177/0018578717750094
Chen J, Xie L, Hu Y, Lan X, Jiang L (2018) Nonconvulsive status epilepticus after cessation of convulsive status epilepticus in pediatric intensive care unit patients. Epilepsy Behav 82:68–73. https://doi.org/10.1016/j.yebeh.2018.02.008
Baselt RC (2017) Disposition of toxic drugs and chemicals in man, 11th edn. Biomedical Publications, Seal Beach, pp 1432–1434, 1810–1812
Levy RJ (2010) Clinical effects and lethal and forensic aspects of propofol. J Forensic Sci 56:S142–S147. https://doi.org/10.1111/j.1556-4029.2010.01583.x
Kranioti EF, Mavroforou A, Mylonakis P, Michalodimitrakis M (2007) Lethal self administration of propofol (Diprivan). A case report and review of the literature. Forensic Sci Int 167:56–58. https://doi.org/10.1016/j.forsciint.2005.12.027
Cirimele V, Kintz P, Doray S, Ludes B (2002) Determination of chronic abuse of the anaesthetic agents midazolam and propofol as demonstrated by hair analysis. Int J Legal Med 116:54–57. https://doi.org/10.1007/s004140100240
Colucci AP, Gagliano-Candela R, Aventaggiato L, De Donno A, Leonardi S, Strisciullo G, Introna F (2013) Suicide by self-administration of a drug mixture (propofol, midazolam, and zolpidem) in an anesthesiologist: the first case report in Italy. J Forensic Sci 58:837–841. https://doi.org/10.1111/1556-4029.12053
Guntner AS, Stöcklegger S, Kneidinger M, Illievich U, Buchberger W (2019) Development of a highly sensitive gas chromatography-mass spectrometry method preceded by solid-phase microextraction for the analysis of propofol in low-volume cerebral microdialysate samples. J Sep Sci 42:1257–1264. https://doi.org/10.1002/jssc.201801066(open access article)
Álvarez-Freire I, Brunetti P, Cabarcos-Fernández P, Fernández-Liste A, Tabernero-Duque MJ, Bermejo-Barrera AM (2018) Determination of benzodiazepines in pericardial fluid by gas chromatography-mass spectrometry. J Pharm Biomed Anal 159:45–52. https://doi.org/10.1016/j.jpba.2018.06.039
Peters FT, Jung J, Kraemer T, Maurer HH (2005) Fast, simple, and validated gas chromatographic-mass spectrometric assay for quantification of drugs relevant to diagnosis of brain death in human blood plasma samples. Ther Drug Monit 27:334–344. https://doi.org/10.1097/01.ftd.0000158079.53577.46
Rochani A, Lam E, Tanjuakio J, Hirose H, Kraft WK, Kaushal G (2020) Simultaneous quantitative LC-MS method of ketamine, midazolam and their metabolites (dehydronorketamine, norketamine and 1hydroxymidazolam) for its application in patients on extracorporeal membrane oxygenation (ECMO) therapy. J Pharm Biomed Anal 178:112947. https://doi.org/10.1016/j.jpba.2019.112947
Zheng Z, Zhang S, Ma W, Zhang L, Huang L, Huang W, Huang M, Wang Z, Li J (2018) Determination of dexmedetomidine by UHPLC–MS/MS and its application to evaluate the effect of dexmedetomidine concentration on the target-controlled infusion concentration of propofol. J Pharm Biomed Anal 154:438–443. https://doi.org/10.1016/j.jpba.2018.03.015
Chen M, Lu W, Lu Y, Kang L, Zhao H, Lin ZJ, Wang W, Fraier D, Ottaviani G (2017) An ultra-sensitive LC-MS/MS method to determine midazolam levels in human plasma: development, validation and application to a clinical study. Bioanalysis 9:297–312. https://doi.org/10.4155/bio-2016-0191
Kang MG, Lin HR (2019) Systematic evaluation and validation of benzodiazepines confirmation assay using LC-MS-MS. J Anal Toxicol 43:96–103. https://doi.org/10.1093/jat/bky071
Cohen S, Lhuillier F, Mouloua Y, Vignal B, Favetta P, Guitton J (2007) Quantitative measurement of propofol and in main glucuroconjugate metabolites in human plasma using solid phase extraction-liquid chromatography-tandem mass spectrometry. J Chromatogr B 854:165–172. https://doi.org/10.1016/j.jchromb.2007.04.021
Lehotay SJ (2011) QuEChERS sample preparation approach for mass spectrometric analysis of pesticide residues in foods. Methods Mol Biol 747:65–91. https://doi.org/10.1007/978-1-61779-136-9_4
Caldas SS, Bolzan CM, Cerqueira MB, Tomasini D, Furlong EB, Fagundes C, Primel EG (2011) Evaluation of a modified QuEChERS extraction of multiple classes of pesticides from a rice paddy soil by LC-APCI-MS/MS. J Agric Food Chem 59:11918–11926. https://doi.org/10.1021/jf202878s
de Oliveira Arias JL, Rombaldi C, Caldas SS, Primel EG (2014) Alternative sorbents for the dispersive solid-phase extraction step in quick, easy, cheap, effective, rugged and safe method for extraction of pesticides from rice paddy soils with determination by liquid chromatography tandem mass spectrometry. J Chromatogr A 1360:66–75. https://doi.org/10.1016/j.chroma.2014.07.082
de Matos EMC, Ribeiro LC, Prestes OD, da Silva JAG, de Farias BS, de A Pinto LA, Zanella R (2019) Multiclass method for the determination of pesticide residues in oat using modified QuEChERS with alternative sorbent and liquid chromatography with tandem mass spectrometry. Food Anal Methods 12:2835–2844. https://doi.org/10.1007/s12161-019-01641-1
Kusano M, Sakamoto Y, Natori Y, Miyagawa H, Tsuchihashi H, Ishii A, Zaitsu K (2019) Development of "quick-DB forensic": a total workflow from QuEChERS-dSPE method to GC–MS/MS quantification of forensically relevant drugs and pesticides in whole blood. Forensic Sci Int 300:125–135. https://doi.org/10.1016/j.forsciint.2019.03.048
Sweeney C, Park Y, Kim JS (2019) Comparison of sample preparation approaches and validation of an extraction method for nitrosatable pesticides and metabolites in human serum and urine analyzed by liquid chromatography-Orbital ion trap mass spectrometry. J Chromatogr A 1603:83–91. https://doi.org/10.1016/j.chroma.2019.06.065
Hasegawa K, Wurita A, Nozawa H, Yamagishi I, Minakata K, Watanabe K, Suzuki O (2018) Fatal zolpidem poisoning due to its intravenous self-injection: postmortem distribution/redistribution of zolpidem and its predominant metabolite zolpidem phenyl-4-carboxylic acid in body fluids and solid tissues in an autopsy case. Forensic Sci Int 290:111–120. https://doi.org/10.1016/j.forsciint.2018.06.044
Robin T, Barnes A, Dulaurent S, Loftus N, Baumgarten S, Moreau S, Marquet P, El Balkhi S, Saint-Marcoux F (2018) Fully automated sample preparation procedure to measure drugs of abuse in plasma by liquid chromatography tandem mass spectrometry. Anal Bioanal Chem 410:5071–5083. https://doi.org/10.1007/s00216-018-1159-7
Mizuno S, Lee X-P, Fujishiro M, Matsuyama T, Yamada M, Sakamoto Y, Kusano M, Zaitsu K, Hasegawa C, Hasegawa I, Kumazawa T, Ishii A, Sato K (2018) High-throughput determination of valproate in human samples by modified QuEChERS extraction and GC–MS/MS. Leg Med 31:66–73. https://doi.org/10.1016/j.legalmed.2018.01.002
Anzillotti L, Odoardi S, Strano-Rossi S (2014) Cleaning up blood samples using a modified “QuEChERS” procedure for the determination of drugs of abuse and benzodiazepines by UPLC–MSMS. Forensic Sci Int 243:99–106. https://doi.org/10.1016/j.forsciint.2014.05.005
Murakami T, Iwamuro Y, Ishimaru R, Chinaka S, Sugimura N, Takayama N (2016) Differentiation of AB-FUBINACA positional isomers by the abundance of product ions using electron ionization-triple quadrupole mass spectrometry. J Mass Spectrom 51:1016–1022. https://doi.org/10.1002/jms.3814
Kohyama E, Chikumoto T, Furukawa R, Suenami K, Kawashima H, Tada H, Nagai H, Soda M, Kitaichi K, Ito T (2017) Regioisomeric differentiation of synthetic cannabinoids with an N-fluorobenzyl indole core by gas chromatography–tandem mass spectrometry. Forensic Chem 6:28–35. https://doi.org/10.1016/j.forc.2017.09.002
Mochizuki A, Nakazawa H, Adachi N, Takekawa K, Shojo H (2018) Identification and quantification of mepirapim and acetyl fentanyl in authentic human whole blood and urine samples by GC–MS/MS and LC-MS/MS. Forensic Toxicol 36:81–87. https://doi.org/10.1007/s11419-017-0384-7
Dybowski MP, Dawidowicz AL (2018) Application of the QuEChERS procedure for analysis of Δ9-tetrahydrocannabinol and its metabolites in authentic whole blood samples by GC–MS/MS. Forensic Toxicol 36:415–423. https://doi.org/10.1007/s11419-018-0419-8
U.S. Food and Drug Administration (2019) Guidance for industry: bioanalytical method validation. https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm. Accessed 21 Jan 2020
Woźniak MK, Banaszkiewicz L, Wiergowski M, Tomczak E, Kata M, Szpiech B, Namieśnik J, Biziuk M (2020) Development and validation of a GC–MS/MS method for the determination of 11 amphetamines and 34 synthetic cathinones in whole blood. Forensic Toxicol 38:42–58. https://doi.org/10.1007/s11419-019-00485-y
Acknowledgments
This study was supported in part by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS), KAKENHI grant (C) 24590865.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest associated with this manuscript.
Ethical approval
This study was approved by the Ethics Committee of Showa University School of Medicine, and informed consent was obtained from the family of the patient prior to participation in this study (No. 2715). The collection of blank blood samples from healthy volunteers was approved by the Ethics Committees of Showa University School of Medicine (No. 1249).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kaki, Y., Fujishiro, M., Lee, XP. et al. Sensitive determination of midazolam and propofol in human plasma by GC–MS/MS. Forensic Toxicol 38, 409–419 (2020). https://doi.org/10.1007/s11419-020-00529-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11419-020-00529-8