Abstract
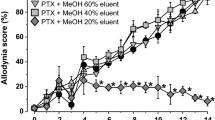
Chemotherapy-induced peripheral neuropathy (CIPN) manifests as mechanical allodynia and hyperalgesia, and is one of the main adverse effects of chemotherapeutic agents. Currently available therapeutic drugs are not sufficiently effective for the management of this adverse effect in the clinic. Therefore, the development of novel therapeutic agents for treating CIPN is necessary. Our previous study suggested the potential of aucubin and pedicularis-lactone (1) as active compounds responsible for the anti-allodynic property of Plantaginis Semen. However, the activity of purified 1 has not been evaluated due to its low content in Plantaginis Semen. In the present study, 1 was isolated from Viticis Fructus, as well as viteoid I (2) and viteoid II (3) during the process of isolation. The purities of isolated 1, 2, and 3 were determined as 67.15%, 92.12%, and 86.72%, respectively, by quantitative 1H-NMR, using DSS-d6 as an internal standard. Repeated daily oral administration of these three iridoids at a dose of 15 mg/kg significantly inhibited the PTX-induced mechanical allodynia in mice, suggesting the anti-allodynic activities of 1, 2, and 3. This study provides confirmatory evidence for the anti-allodynic activity of purified 1 and also reveals two additional active iridoids from Viticis Fructus. These three iridoids could be potential candidates for the treatment of CIPN.




Similar content being viewed by others
References
Lolignier S, Eijkelkamp N, Wood JN (2015) Mechanical allodynia. Pflugers Arch 467:133–139. https://doi.org/10.1007/s00424-014-1532-0
Boland BA, Sherry V, Polomano RC (2010) Chemotherapy induced peripheral neuropathy in cancer survivors. Oncology. https://www.cancernetwork.com/view/chemotherapy-induced-peripheral-neuropathy-cancer-survivors. Accessed 3 Aug 2020
Miltenburg NC, Boogerd W (2014) Chemotherapy-induced neuropathy: a comprehensive survey. Cancer Treat Rev 40:872–882. https://doi.org/10.1016/j.ctrv.2014.04.004
Seretny M, Currie GL, Sena ES, Ramnarine S, Grant R, MacLeod MR, Colvin LA, Fallon M (2014) Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Pain 155:2461–2470. https://doi.org/10.1016/j.pain.2014.09.020
Andoh T, Kitamura R, Fushimi H, Komatsu K, Shibahara N, Kuraishi Y (2014) Effects of goshajinkigan, hachimijiogan, and rokumigan on mechanical allodynia induced by Paclitaxel in mice. J Tradit Complement Med 4:293–297. https://doi.org/10.4103/2225-4110.128906
Andoh T, Kato M, Kitamura R, Mizoguchi S, Uta D, Toume K, Komatsu K, Kuraishi Y (2016) Prophylactic administration of an extract from Plantaginis Semen and its major component aucubin inhibits mechanical allodynia caused by paclitaxel in mice. J Tradit Complement Med 6:305–308. https://doi.org/10.1016/j.jtcme.2015.12.001
Toume K, Hou ZY, Yu HH, Kato M, Maesaka M, Bai YJ, Hanazawa S, Ge YW, Andoh T, Komatsu K (2019) Search of anti-allodynic compounds from Plantaginis Semen, a crude drug ingredient of Kampo formula "Goshajinkigan". J Nat Med 73:761–768. https://doi.org/10.1007/s11418-020-01404-x
Ono M, Ito Y, Kubo S, Nohara T (1997) Two new iridoids from Viticis trifoliae fructus. Chem Pharm Bull 45:1094–1096. https://doi.org/10.1248/cpb.45.1094
Ma QJ, Han L, Mu Y, Guan PP, Lei H, Wang ZY, Huang XS (2017) New iridoids from Scrophularia ningpoensis. Chem Pharm Bull 65:869–873. https://doi.org/10.1248/cpb.c17-00163
Yang L, Wang CZ, Jia ZJ (1995) Iridoids in roots of Pedicularis chinensis. Phytochemistry 40:491–494. https://doi.org/10.1016/0031-9422(95)00354-a
Review Committee of the Japanese standards for non-Pharmacopoeial crude drugs (2015) The Japanese standards for non-pharmacopoeial crude drugs 2015: bilingual in Japanese and English. Yakuji Nippo Ltd., Tokyo, pp 182–183
Kimura K, Kimura T (1981) Medicinal plants of Japan in color. Hoikusha Publishing Co., Ltd., Osaka, p 183
Lee C, Lee JW, Jin Q, Lee HJ, Lee SJ, Lee D, Lee MK, Lee CK, Hong JT, Hwang BY (2013) Anti-inflammatory constituents from the fruits of Vitex rotundifolia. Bioorg Med Chem Lett 23:6010–6014. https://doi.org/10.1016/j.bmcl.2013.08.004
Wang WQ, Yin YP, Jun L, Xuan LJ (2018) Halimane-type diterpenoids from Vitex rotundifolia and their anti-hyperlipidemia activities. Phytochemistry 146:56–62. https://doi.org/10.1016/j.phytochem.2017.12.002
Kim A, Im M, Ma JY (2017) SRVF a novel herbal formula including Scrophulariae radix and Viticis fructus, disrupts focal adhesion and causes detachment-induced apoptosis in malignant cancer cells. Sci Rep 7:12756. https://doi.org/10.1038/s41598-017-12934-y
Hu Y, Xin HL, Zhang QY, Zheng HC, Rahman K, Qin LP (2007) Anti-nociceptive and anti-hyperprolactinemia activities of fructus Viticis and its effective fractions and chemical constituents. Phytomedicine 14:668–674. https://doi.org/10.1016/j.phymed.2007.01.008
Okuyama E, Fujimori S, Yamazaki M, Deyama T (1998) Pharmacologically active components of viticis fructus (Vitex rotundifolia). II. The components having analgesic effects. Chem Pharm Bull 46:655–662. https://doi.org/10.1248/cpb.46.655
Rani A, Sharma A (2013) The genus Vitex: a review. Pharmacogn Rev 7:188–198. https://doi.org/10.4103/0973-7847.120522
Bello MO, Zaki AA, Aloko S, Fasinu PS, Bello EO, Ajao UL, Oguntoye OS (2018) The genus Vitex: an overview of iridoids as chemotaxonomic marker. Beni-Suef Univ J Basic Appl Sci 7:414–419. https://doi.org/10.1016/j.bjbas.2017.07.004
Tiwari N, Luqman S, Masood N, Gupta MM (2012) Validated high performance thin layer chromatographic method for simultaneous quantification of major iridoids in Vitex trifolia and their antioxidant studies. J Pharm Biomed Anal 61:207–214. https://doi.org/10.1016/j.jpba.2011.12.007
Hu Y, Hou TT, Zhang QY, Xin HL, Zheng HC, Rahman K, Qin LP (2007) Evaluation of the estrogenic activity of the constituents in the fruits of Vitex rotundifolia L. for the potential treatment of premenstrual syndrome. J Pharm Pharmacol 59:1307–1312. https://doi.org/10.1211/jpp.59.9.0016
Pandey A, Bani S, Satti NK, Gupta BD, Suri KA (2012) Anti-arthritic activity of agnuside mediated through the down-regulation of inflammatory mediators and cytokines. Inflamm Res 61:293–304. https://doi.org/10.1007/s00011-011-0410-x
Gauchan P, Andoh T, Kato A, Sasaki A, Kuraishi Y (2009) Effects of the prostaglandin E1 analog limaprost on mechanical allodynia caused by chemotherapeutic agents in mice. J Pharmacol Sci 109:469–472. https://doi.org/10.1254/jphs.08325sc
Yamate J, Sakamori M, Kuwamura M, Kotani T (2007) Neoplastic and non-neoplastic cell lines from a malignant peripheral nerve sheath tumour of the cervix of a rat. J Comp Pathol 137:9–21. https://doi.org/10.1016/j.jcpa.2007.03.004
Andoh T, Uta D, Kato M, Toume K, Komatsu K, Kuraishi Y (2017) Prophylactic administration of aucubin inhibits paclitaxel-induced mechanical allodynia via the inhibition of endoplasmic reticulum stress in peripheral Schwann cells. Biol Pharm Bull 40:473–478. https://doi.org/10.1248/bpb.b16-00899
Wang YB, Zhang RZ, Xie JQ, Lu JZ, Yue ZJ (2014) Analgesic activity of catalpol in rodent models of neuropathic pain, and its spinal mechanism. Cell Biochem Biophys 70:1565–1571. https://doi.org/10.1007/s12013-014-0096-0
Zheng YA, Yin XF, Huo FQ, Xiong H, Mei ZN (2015) Analgesic effects and possible mechanisms of iridoid glycosides from Lamiophlomis rotata (Benth.) Kudo in rats with spared nerve injury. J Ethnopharmacol 173:204–211. https://doi.org/10.1016/j.jep.2015.06.045
Lu Y, Yao JY, Gong CL, Wang B, Zhou P, Zhou SL, Yao XH (2018) Gentiopicroside ameliorates diabetic peripheral neuropathy by modulating PPAR-γ/AMPK/ACC signaling pathway. Cell Physiol Biochem 50:585–596. https://doi.org/10.1159/000494174
Kimura T, But PP, Guo JX, Sung CK (1996) International collation of traditional and folk medicine. World Scientific, Singapore, pp 141–142
Peng QH, Han ZZ, Tong RC, Hu XQ, Yang QM, Qi M, Shi YH, Wang ZT, Yang L (2017) Chemical constituents from hypoglycemic active part of Plantaginis Semen. Zhongguo Zhong Yao Za Zhi 42:4150–4153. https://doi.org/10.19540/j.cnki.cjcmm.20170905.009(Article in Chinese)
Leitão SG, Santos TCd, Monache FD, Matheus ME, Fernandes PD, Marinho BG (2011) Phytochemical profile and analgesic evaluation of Vitex cymosa leaf extracts. Rev Bras Farmacogn Braz J Pharmacogn 21:874–883. https://doi.org/10.1590/s0102-695x2011005000160
Kaneko T, Ohtani K, Kasai R, Yamasaki K, Nguyen M (1997) Iridoids and iridoid glucosides from fruits of Crescentia cujete. Phytochemistry 46:907–910. https://doi.org/10.1016/s0031-9422(97)00375-0
Davini E, Iavarone C, Trogolo C (1987) Iridoid glucosides as sources of cyclopentanoid 1,5-dialdehydes: conversion of aucubigenin to aucommial and eucommiol. Phytochemistry 26:1449–1451. https://doi.org/10.1016/s0031-9422(00)81832-4
Ueda H (2008) Peripheral mechanisms of neuropathic pain-involvement of lysophosphatidic acid receptor-mediated demyelination. Mol Pain 4:11. https://doi.org/10.1186/1744-8069-4-11
Komiya Y (1992) Changes of fast axonal transport by taxol injected subepineurally into the rat sciatic nerve. Neurosci Res 14:159–165. https://doi.org/10.1016/0168-0102(92)90077-p
Cavaletti G, Tredici G, Braga M, Tazzari S (1995) Experimental peripheral neuropathy induced in adult rats by repeated intraperitoneal administration of taxol. Exp Neurol 133:64–72. https://doi.org/10.1006/exnr.1995.1008
Liao PC, Tan SK, Lieu CH, Jung HK (2008) Involvement of endoplasmic reticulum in paclitaxel-induced apoptosis. J Cell Biochem 104:1509–1523. https://doi.org/10.1002/jcb.21730
Tanimukai H, Kanayama D, Omi T, Takeda M, Kudo T (2013) Paclitaxel induces neurotoxicity through endoplasmic reticulum stress. Biochem Biophys Res Commun 437:151–155. https://doi.org/10.1016/j.bbrc.2013.06.057
Zhuang ZY, Gerner P, Woolf CJ, Ji RR (2005) ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain 114:149–159. https://doi.org/10.1016/j.pain.2004.12.022
Okada-Ogawa A, Suzuki I, Sessle BJ, Chiang CY, Salter MW, Dostrovsky JO, Tsuboi Y, Kondo M, Kitagawa J, Kobayashi A, Noma N, Imamura Y, Iwata K (2009) Astroglia in medullary dorsal horn (trigeminal spinal subnucleus caudalis) are involved in trigeminal neuropathic pain mechanisms. J Neurosci 29:11161–11171. https://doi.org/10.1523/jneurosci.3365-09.2009
Nakagawa T, Kaneko S (2010) Spinal astrocytes as therapeutic targets for pathological pain. J Pharmacol Sci 114:347–353. https://doi.org/10.1254/jphs.10r04cp
Zhang HJ, Yoon SY, Zhang HM, Dougherty PM (2012) Evidence that spinal astrocytes but not microglia contribute to the pathogenesis of Paclitaxel-induced painful neuropathy. J Pain 13:293–303. https://doi.org/10.1016/j.jpain.2011.12.002
Makker PG, Duffy SS, Lees JG, Perera CJ, Tonkin RS, Butovsky O, Park SB, Goldstein D, Moalem-Taylor G (2017) Characterisation of immune and neuroinflammatory changes associated with chemotherapy-induced peripheral neuropathy. PLoS One 12:e0170814. https://doi.org/10.1371/journal.pone.0170814
Liu XJ, Tonello R, Ling YJ, Gao YJ, Berta T (2019) Paclitaxel-activated astrocytes produce mechanical allodynia in mice by releasing tumor necrosis factor-α and stromal-derived cell factor 1. J Neuroinflamm 16:209. https://doi.org/10.1186/s12974-019-1619-9
Acknowledgements
This study was supported in part by a Grant-in-Aid for the Cooperative Research Project from the Institute of Natural Medicine, University of Toyama in 2016 and 2017; JSPS KAKENHI Grant numbers 15K07993, 15H05268 and 18K06728; Wakanyaku-Biotechnology Research Grant from Toyama prefecture; and JSPS Core-to-Core Program, Asia-Africa Science Platforms. The authors would like to thank Nippon Funmatsu Yakuhin Co., LTD, for providing the extract of Viticis Fructus in large scale.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yu, H., Toume, K., Kurokawa, Y. et al. Iridoids isolated from Viticis Fructus inhibit paclitaxel-induced mechanical allodynia in mice. J Nat Med 75, 48–55 (2021). https://doi.org/10.1007/s11418-020-01441-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-020-01441-6