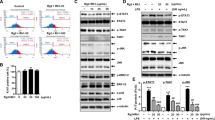
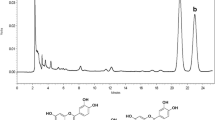
Abstract
The aim of this study was to investigate the effects of two structurally related flavonoids found in Cyclopia subternata, vicenin-2 (VCN) and scolymoside (SCL) on lipopolysaccharide (LPS)-induced liver failure in mice and to elucidate underlying mechanisms. Mice were treated intravenously with VCN or SCL at 12 h after LPS treatment. LPS significantly increased mortality, serum levels of alanine transaminase, aspartate transaminase, and inflammatory cytokines, and toll-like receptor 4 (TLR4) protein expression; these effects of LPS were inhibited by VCN or SCL. It also attenuated the LPS-induced activation of myeloid differentiation primary response gene 88 and TLR-associated activator of interferon-dependent signaling pathways of the TLR system. Our results suggest that VCN or SCL protects against LPS-induced liver damage by inhibiting the TLR-mediated inflammatory pathway, indicating its potential to treat liver diseases.





Similar content being viewed by others
References
Liu F, Lin Y, Li Z, Ma X, Han Q, Liu Y, Zhou Q, Liu J, Li R, Li J, Gao L (2014) Glutathione S-transferase A1 (GSTA1) release, an early indicator of acute hepatic injury in mice. Food Chem Toxicol 71:225–230
Wu Z, Han M, Chen T, Yan W, Ning Q (2010) Acute liver failure: mechanisms of immune-mediated liver injury. Liver Int 30:782–794
Kato Y, Morikawa A, Sugiyama T, Koide N, Jiang GZ, Takahashi K, Yokochi T (1995) Role of tumor necrosis factor-alpha and glucocorticoid on lipopolysaccharide (LPS)-induced apoptosis of thymocytes. FEMS Immunol Med Microbiol 12:195–204
Kosai K, Matsumoto K, Funakoshi H, Nakamura T (1999) Hepatocyte growth factor prevents endotoxin-induced lethal hepatic failure in mice. Hepatology 30:151–159
Prior RL, Cao G (1999) Antioxidant capacity and polyphenolic components of teas: implications for altering in vivo antioxidant status. Proc Soc Exp Biol Med 220:255–261
Warren CP (1999) Antioxidant effects of herbs. Lancet 353:676
Joubert E, Joubert ME, Bester C, De Beer D, De Lange JH (2011) Honeybush (Cyclopia spp.): from local cottage industry to global markets—the catalytic and supporting role of research. S Afr J Bot 77:889–907
Islam MN, Ishita IJ, Jung HA, Choi JS (2014) Vicenin 2 isolated from Artemisia capillaris exhibited potent anti-glycation properties. Food Chem Toxicol 69:55–62
Sanchez GM, Re L, Giuliani A, Nunez-Selles AJ, Davison GP, Leon-Fernandez OS (2000) Protective effects of Mangifera indica L. extract, mangiferin and selected antioxidants against TPA-induced biomolecules oxidation and peritoneal macrophage activation in mice. Pharmacol Res 42:565–573
Leiro JM, Alvarez E, Arranz JA, Siso IG, Orallo F (2003) In vitro effects of mangiferin on superoxide concentrations and expression of the inducible nitric oxide synthase, tumour necrosis factor-alpha and transforming growth factor-beta genes. Biochem Pharmacol 65:1361–1371
Pardo Andreu GL, Maurmann N, Reolon GK, de Farias CB, Schwartsmann G, Delgado R, Roesler R (2010) Mangiferin, a naturally occurring glucoxilxanthone improves long-term object recognition memory in rats. Eur J Pharmacol 635:124–128
Jack BU, Malherbe CJ, Huisamen B, Gabuza K, Mazibuko-Mbeje S, Schulze AE, Joubert E, Muller CJF, Louw J, Pheiffer C (2017) A polyphenol-enriched fraction of Cyclopia intermedia decreases lipid content in 3T3-L1 adipocytes and reduces body weight gain of obese db/db mice. S Afr J Bot 110:216–229
Marrassini C, Davicino R, Acevedo C, Anesini C, Gorzalczany S, Ferraro G (2011) Vicenin-2, a potential anti-inflammatory constituent of Urtica circularis. J Nat Prod 74:1503–1507
Lee W, Yoon EK, Kim KM, Park DH, Bae JS (2015) Antiseptic effect of vicenin-2 and scolymoside from Cyclopia subternata (honeybush) in response to HMGB1 as a late sepsis mediator in vitro and in vivo. Can J Physiol Pharmacol 93:709–720
Lee W, Ku SK, Bae JS (2015) Ameliorative effect of vicenin-2 and scolymoside on TGFBIp-induced septic responses. Inflammation 38:2166–2177
Kang H, Ku SK, Jung B, Bae JS (2015) Anti-inflammatory effects of vicenin-2 and scolymoside in vitro and in vivo. Inflamm Res 64:1005–1021
Lee IC, Bae JS (2016) Anti-inflammatory effects of vicenin-2 and scolymoside on polyphosphate-mediated vascular inflammatory responses. Inflamm Res 65:203–212
Lee I-C, Bae J-S (2015) Inhibitory effect of vicenin-2 and scolymoside on secretory group IIA phospholipase A2. Anim Cells Syst 19:305–311
Lee W, Bae JS (2015) Antithrombotic and antiplatelet activities of vicenin-2. Blood Coagul Fibrinolysis 26:628–634
Yoon EK, Ku SK, Lee W, Kwak S, Kang H, Jung B, Bae JS (2015) Antitcoagulant and antiplatelet activities of scolymoside. BMB Rep 48:577–582
Ku SK, Bae JS (2016) Vicenin-2 and scolymoside inhibit high-glucose-induced vascular inflammation in vitro and in vivo. Can J Physiol Pharmacol 94:287–295
Lee Y, Jeong GS, Kim KM, Lee W, Bae JS (2018) Cudratricusxanthone A attenuates sepsis-induced liver injury via SIRT1 signaling. J Cell Physiol 233:5441–5446
Kirschning CJ, Bauer S (2001) Toll-like receptors: cellular signal transducers for exogenous molecular patterns causing immune responses. Int J Med Microbiol 291:251–260
Horner AA, Redecke V, Raz E (2004) Toll-like receptor ligands: hygiene, atopy and therapeutic implications. Curr Opin Allergy Clin Immunol 4:555–561
Kutikhin AG (2011) Impact of toll-like receptor 4 polymorphisms on risk of cancer. Hum Immunol 72:193–206
Brown J, Wang H, Hajishengallis GN, Martin M (2011) TLR-signaling networks: an integration of adaptor molecules, kinases, and cross-talk. J Dent Res 90:417–427
Cook DN, Pisetsky DS, Schwartz DA (2004) Toll-like receptors in the pathogenesis of human disease. Nat Immunol 5:975–979
Patterson H, Nibbs R, McInnes I, Siebert S (2014) Protein kinase inhibitors in the treatment of inflammatory and autoimmune diseases. Clin Exp Immunol 176:1–10
Ben Ari Z, Avlas O, Pappo O, Zilbermints V, Cheporko Y, Bachmetov L, Zemel R, Shainberg A, Sharon E, Grief F, Hochhauser E (2012) Reduced hepatic injury in toll-like receptor 4-deficient mice following D-galactosamine/lipopolysaccharide-induced fulminant hepatic failure. Cell Physiol Biochem 29:41–50
Turner MD, Nedjai B, Hurst T, Pennington DJ (2014) Cytokines and chemokines: at the crossroads of cell signalling and inflammatory disease. Biochim Biophys Acta 1843:2563–2582
Song GY, Chung CS, Jarrar D, Chaudry IH, Ayala A (2001) Evolution of an immune suppressive macrophage phenotype as a product of P38 MAPK activation in polymicrobial sepsis. Shock 15:42–48
Wang X, Qin W, Song M, Zhang Y, Sun B (2016) Exogenous carbon monoxide inhibits neutrophil infiltration in LPS-induced sepsis by interfering with FPR1 via p38 MAPK but not GRK2. Oncotarget 7:34250–34265
Sato S, Sugiyama M, Yamamoto M, Watanabe Y, Kawai T, Takeda K, Akira S (2003) Toll/IL-1 receptor domain-containing adaptor inducing IFN-beta (TRIF) associates with TNF receptor-associated factor 6 and TANK-binding kinase 1, and activates two distinct transcription factors, NF-kappa B and IFN-regulatory factor-3, in the Toll-like receptor signaling. J Immunol 171:4304–4310
Fitzgerald KA, McWhirter SM, Faia KL, Rowe DC, Latz E, Golenbock DT, Coyle AJ, Liao SM, Maniatis T (2003) IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol 4:491–496
David S, Ghosh CC, Kumpers P, Shushakova N, Van Slyke P, Khankin EV, Karumanchi SA, Dumont D, Parikh SM (2011) Effects of a synthetic PEG-ylated Tie-2 agonist peptide on endotoxemic lung injury and mortality. Am J Physiol Lung Cell Mol Physiol 300:L851–L862
Fu X, Ju J, Lin Z, Xiao W, Li X, Zhuang B, Zhang T, Ma X, Ma C, Su W, Wang Y, Qin X, Liang S (2016) Target deletion of complement component 9 attenuates antibody-mediated hemolysis and lipopolysaccharide (LPS)-induced acute shock in mice. Sci Rep 6:30239
Ku SK, Baek MC, Bae JS (2015) Anti-inflammatory effects of methylthiouracil in vitro and in vivo. Toxicol Appl Pharmacol 288:374–386
Lee W, Lee Y, Jeong GS, Ku SK, Bae JS (2017) Cudratricusxanthone A attenuates renal injury in septic mice. Food Chem Toxicol 106:404–410
Ozdulger A, Cinel I, Koksel O, Cinel L, Avlan D, Unlu A, Okcu H, Dikmengil M, Oral U (2003) The protective effect of N-acetylcysteine on apoptotic lung injury in cecal ligation and puncture-induced sepsis model. Shock 19:366–372
Lee W, Ku SK, Kim JE, Cho GE, Song GY, Bae JS (2019) Pulmonary protective functions of rare ginsenoside Rg4 on particulate matter-induced inflammatory responses. Biotechnol Bioprocess Eng 24:445–453
Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN et al (1995) Histological grading and staging of chronic hepatitis. J Hepatol 22:696–699
Jonsson JR, Clouston AD, Ando Y, Kelemen LI, Horn MJ, Adamson MD, Purdie DM, Powell EE (2001) Angiotensin-converting enzyme inhibition attenuates the progression of rat hepatic fibrosis. Gastroenterology 121:148–155
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lee, IC., Bae, JS. Hepatoprotective effects of vicenin-2 and scolymoside through the modulation of inflammatory pathways. J Nat Med 74, 90–97 (2020). https://doi.org/10.1007/s11418-019-01348-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-019-01348-x