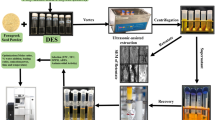
Abstract
1H NMR-based metabolomics has been applied in research on food, herbal medicine, and natural products. Although excellent results were reported, samples were directly extracted with a deuterated solvent (e.g., methanol-d4 or D2O) in most reports. As primary metabolites account for most of the results, data for secondary metabolites are partially reflected. Consequently, secondary metabolites tend to be excluded from factor loading analysis, serving as a significant unfavorable feature of 1H NMR-based metabolomics when investigating biologically active or functional components in natural products and health foods. Reversed-phase solid-phase extraction column (RP-SPEC) was applied for sample preparation in 1H NMR-based metabolomics to overcome this feature. The methanol extract from Saposhnikoviae radix (SR), an important crude drug, was fractionated with RP-SPEC into 5% methanol-eluting fractions, and the remaining fraction was collected. Each fraction was subjected to 1H NMR-based metabolomics and compared to results from conventional 1H NMR-based metabolomics. Based on principal component analysis (PCA) and partial least squares projections to latent structures discriminant analysis (PLS-DA), the 5% methanol fraction and conventional method reflected the amount of saccharides such as sucrose on the PC1/PLS1 axes, and wild and cultivated samples were discriminated along those axes. The remaining fraction clearly distinguished SR from Peucedanum ledebourielloides root. The compounds responsible for this discrimination were deemed falcarindiol derivatives and other unidentified secondary metabolites from the s-plot on PLS-DA. The secondary metabolites from original plants were, therefore, presumed to be concentrated in the remaining fraction by RP-SPEC treatment and strongly reflected the species differences. The developed series is considered effective to perform quality evaluation of crude drugs and natural products.
Graphic abstract







Similar content being viewed by others
References
Song H, Ryu HW, Lee KJ, Jeong IY, Kim DS, Oh S (2014) Metabolomics investigation of flavonoid synthesis in soybean leaves depending on the growth stage. Metabolomics 10(5):833–841
Lawal U, Maulidiani M, Shaari K, Ismail IS, Khatib A, Abas F (2015) Metabolite profiling of Ipomoea aquatica at different growth stages in correlation to the antioxidant and α-glucosidase inhibitory activities elucidated by 1H NMR-based metabolomics. Sci Hort 192:400–408
Huo Y, Kamal GM, Wang J, Liu H, Zhang G, Hu Z, Anwar F, Du H (2017) 1H NMR-based metabolomics for discrimination of rice from different geographical origins of China. J Cereal Sci 76:243–252
Fujimura Y, Kurihara K, Ida M, Kosaka R, Miura D, Wariishi H, Maeda-Yamamoto M, Nesumi A, Saito T, Kanda T, Yamada K, Tachibana H (2011) Metabolomics-driven nutraceutical evaluation of diverse green tea cultivars. PLOS One 6(8):e23426
Tianniam S, Tarachiwin L, Bamba T, Kobayashi A, Fukusaki E (2008) Metabolic profiling of Angelica acutiloba roots utilizing gas chromatography–time-of-flight–mass spectrometry for quality assessment based on cultivation area and cultivar via multivariate pattern recognition. J Biosci Bioeng 105(6):655–659
Kim HK, Khan S, Wilson EG, Kricun SDP, Meissner A, Goraler S, Deelder AM, Choi YH, Verpoorte R (2010) Metabolic classification of South American Ilex species by NMR-based metabolomics. Phytochemistry 71:773–784
Wei F, Furihata K, Miyakawa T, Tanokura M (2014) A pilot study of NMR-based sensory prediction of roasted coffee bean extracts. Food Chem 152:363–369
Moldes CA, Cantarelli MA, Camiña JM, Tsai SM, Azevedo RA (2017) Changes in amino acid profile in roots of glyphosate resistant and susceptible soybean (Glycine max) induced by foliar glyphosate application. J Agric Food Chem 65(40):8823–8828
Shen Y, Hou J, Deng W, Feng Z, Yang M, Cheng J, Wu W, Guo D (2017) Comparative analysis of ultrafine granular powder and decoction pieces of Salvia miltiorrhiza by UPLC-UV-MSn combined with statistical analysis. Planta Med 83(6):557–564
Pongsuwan W, Fukusaki E, Bamba T, Yonetani T, Yamahara T, Kobayashi A (2007) Prediction of Japanese green tea ranking by gas chromatography/mass spectrometry-based hydrophilic metabolite fingerprinting. J Agric Food Chem 55:231–236
Catelani TA, Santos JR, Páscoa RNMJ, Pezzaa L, Pezza HR, Lopes JA (2018) Real-time monitoring of a coffee roasting process with near infrared spectroscopy using multivariate statistical analysis: a feasibility study. Talanta 179:292–299
Kai H, Kinoshita K, Harada H, Uesawa Y, Maeda A, Suzuki R, Okada Y, Takahash K, Matsuno K (2017) Establishment of a direct-injection electron ionization-mass spectrometry metabolomics method and its application to lichen profiling. Anal Chem 89(12):6408–6414
Kim HK, Choi YH, Verpoorte R (2010) NMR-based metabolomics analysis of plants. Nat Protoc 5(3):536–549
Ali K, Maltese F, Fortes AM, Pais MS, Verpoorte R, Choi YH (2011) Pre-analytical method for NMR-based grape metabolic fingerprinting and chemometrics. Anal Chim Acta 703:179–186
Fraccaroli M, Nicoletti S, Maltese F, Choi YH, Guzzo F, Levi M, Verpoorte R (2008) Pre-analytical method for metabolic profiling of plant cell cultures of Passiflora garckei. Biotechnol Lett 30:2031–2036
The Ministry of Health, Labour and Welfare, Japan (2016) The Japanese Pharmacopoeia, 17th edn. The MHLW Ministerial Notification No. 64, Tokyo, pp 1971–1972 (English version)
Tai J, Cheung S (2007) Anti-proliferative and antioxidant activities of Saposhnikovia divaricata. Oncol Rep 18:227–234
Okuyama E, Hasegawa T, Matsushita T, Fujimoto H, Ishibashi M, Yamazaki M (2001) Analgesic components of Saposhnikovia root (Saposhnikovia divaricata). Chem Pharm Bull 49(2):154–160
Zheng X, Du J, Xu Y, Liao D, Pettit GR (2010) Cytotoxic lipid esters from Peucedanum ledebourielloides. Med Chem Res 19:337–343
Maruyama T, Ezaki M, Shiba M, Yamaji H, Yoshitomi T, Kawano N, Cheng SZX, Yokokura T, Yamamoto Y, Fuchino H, Sun H, Komatsu K, Kawahara N (2018) Botanical origin and chemical constituents of commercial SR and its related crude drugs available in Shaanxi and the surrounding regions. J Nat Med 72:267–273
Nishihara M, Nukui K, Osumi Y, Shiota H (2018) Quality evaluation of Saposhnikoviae radix (Differences between wild-type and cultivated products). Yakugaku Zasshi 138:571–579
Fujioka T, Furumi K, Fujii H, Okabe H, Mihashi K, Nakano Y, Matsunaga H, Katano M, Mori M (1999) Antiproliferative constituents from umbelliferae plants. V. A new furanocoumarin and falcarindiol furanocoumarin ethers from the root of Angelica japonica. Chem Pharm Bull 47(1):96–100
Su X, Li X, Tao H, Zhou J, Wu T, Chou G, Cheng Z (2013) Simultaneous isolation of seven compounds from Glehnia littoralis roots by off-line overpressured layer chromatography guided by a TLC antioxidant autographic assay. J Sep Sci 36(21–22):3644–3650
Miyazawa M, Shimamura H, Bhuva RC, Nakamura S, Kameoka H (1996) Antimutagenic activity of falcarindiol from Peucedanum praeruptorum. J Agric Food Chem 44(11):3444–3448
Gwang L, Hyung-Gu P, Choi M, Kim Y, Park Y, Song K, Cheong C, Bae Y (2000) Falcarindiol, a polyacetylenic compound isolated from Peucedanum japonicum, inhibits mammalian DNA topoisomerase I. J Microbiol Biotechnol 10(3):394–398
Lee J, Lee YJ, Kim J, Bang O (2015) Pyranocoumarins from root extracts of Peucedanum praeruptorum Dunn with multidrug resistance reversal and anti-inflammatory activities. Molecules 20(12):20967–20978
Acknowledgements
This study was supported by a research grant from the Japan Agency for Medical Research and Development (the Grant number: 17ak0101074j2001, 15ak0101017h0003).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflict of interest to declare in this research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yoshitomi, T., Wakana, D., Uchiyama, N. et al. 1H NMR-based metabolomic analysis coupled with reversed-phase solid-phase extraction for sample preparation of Saposhnikovia roots and related crude drugs. J Nat Med 74, 65–75 (2020). https://doi.org/10.1007/s11418-019-01343-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-019-01343-2