Abstract
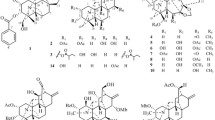
Seven new pregnane glycosides (1–7) and eight known compounds (8–15) were isolated from the bark of Marsdenia cundurango (Asclepiadaceae). The structures of 1–7 were determined by spectroscopic analysis, including two-dimension NMR spectroscopy, chemical transformations, and chromatographic analysis of the hydrolyzed products. The isolated compounds 1–15 were evaluated for their cytotoxic activity against HL-60 human leukemia cells, A549 human lung adenocarcinoma cells, and TIG-3 normal human lung cells, including apoptosis-inducing activity of a representative pregnane glycoside in HL-60 cells.





Similar content being viewed by others
References
The Ministry of Health, Labour and Welfare, Japan. The Japanese pharmacopoeia, 17th edn (Ministry notification no. 64 of March 7, 2016). The Ministry of Health, Labour and Welfare, Tokyo
Tschesche R, Welzel P, Snatzke G (1965) Digitanolglykoside—XII die constitution von kondurangogenin A, dem aglykon eines esterglykosids der kondurangorinde. Tetrahedron 21:1777–1795
Tschesche R, Welzel P, Fehlhaber H (1965) Digitanolglykoside—XIII massenspektrometrische untersuchungen am kondurangogenin A. Tetrahedron 21:1797–1807
Tschesche R, Kohl H, Welzel P (1967) Digitanolglykoside—XVI die struktur der kondurangogenine A und C. Tetrahedron 23:1461–1471
Tschesche R, Kohl H (1968) Digitanolglykoside-XIX die struktur der kondurangoglykoside A, A1 und C, C1. Tetrahedron 24:4359–4371
Hayashi K, Wada K, Mitsuhashi H, Bando H, Takase M, Terada S, Koide Y, Aiba T, Narita T, Mizuno D (1980) Antitumor active glycosides from Condurango cortex. Chem Pharm Bull 28:1954–1958
Hayashi K, Wada K, Mitsuhashi H, Bando H, Takase M, Terada S, Koide Y, Aiba T, Narita T (1981) Further investigation of antitumor condurangoglycosides with C-18 oxygenated aglycone. Chem Pharm Bull 29:2725–2730
Berger S, Junior P, Kopanski L (1988) Structural revision of pregnane ester glycosides from Condurango cortex and new compounds. Phytochemistry 27:1451–1458
Umehara K, Endoh M, Miyase T, Kuroyanagi M, Ueno A (1994) Studies on differentiation inducers. Pregnane derivatives from Condurango cortex. Chem Pharm Bull 42:611–616
Sikdar S, Mukherjee A, Boujedaini N, Khuda-Bukhsh AR (2013) Ethanolic extract of Condurango (Marsdenia condurango) used in traditional systems of medicine including homeopathy against cancer can induce DNA damage and apoptosis in non small lung cancer cells, A549 and H522, in vitro. Tang Humanit Med 3:e9
Sikdar S, Mukherjee A, Ghosh S, Khuda-Bukhsh AR (2014) Codurango glycoside-rich components stimulate DNA damage-induced cell cycle arrest and ROS-mediated caspase-3 dependent apoptosis through inhibition of cell-proliferation in lung cancer, in vitro and in vivo. Environ Toxicol Pharmacol 37:300–331
Yokosuka A, Mimaki Y, Sashida Y (2002) Steroidal and pregnane glycosides from the rhizomes of Tacca chantrieri. J Nat Prod 65:1293–1298
Kubo A, Kuroda M, Yokosuka A, Sakagami H, Mimaki Y (2015) Amurensiosides L-P, five new cardenolide glycosides from the roots of Adonis amurensis. Nat Prod Commun 10:27–32
Yokosuka A, Iguchi T, Kawahata R, Mimaki Y (2018) Cytotoxic bufadienolides from the whole plants of Helleborus foetidus. Phytochem Lett 23:94–99
Zhang Y, Yuan J, Ding W (1993) Structural elucidation of marsdeoreophiside A. Zhongcaoyao 24:171–173
Allgeier H (1968) Struktur der pachybiose und asclepobiose. Helv Chim Acta 51:311–325
Gupta VS, Kumar A, Deepak D, Khare A, Khare NK (2003) Pregnanes and pregnane glycosides from Marsdenia roylei. Phytochemistry 64:1327–1333
Acknowledgements
This work was financially supported in part by the Japan Society for the Promotion of Sciences (JSPS) KAKENHI (Grant number 26860069).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tatsuno, S., Yokosuka, A., Hatsuma, F. et al. Pregnane glycosides from the bark of Marsdenia cundurango and their cytotoxic activity. J Nat Med 73, 93–103 (2019). https://doi.org/10.1007/s11418-018-1248-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-018-1248-0