Abstract
Telomere shortening is a biological aging hallmark. The effect of short telomere length may be targeted by increased physical activity to reduce the risk of multiple aging-related diseases, including coronary heart disease (CHD). The objective was to assess the moderation effect of accelerometer-based physical activity (aPA) on the association between shorter leukocyte telomere length (LTL) relatively in the population sample and incident CHD. Data were from the UK Biobank participants with well-calibrated accelerometer data for at least 6.5 days (n = 54,180). Relative mean LTL at baseline (5–6 years prior to aPA assessment) was measured in T/S ratio, using a multiplex quantitative polymerase chain reaction (qPCR) technology, by comparing the amount of the telomere amplification product (T) to that of a single-copy gene (S). aPA measures included total number of events (at least 10-s continued physical activity > 32 milligravities [mg]), total volume, mean duration, mean intensity, and peak intensity of all events. LTL, aPA measures, and their interactions were associated with incident CHD (mean follow-up 6.8 years) using Cox proportional hazards models adjusting for covariates. Longer LTL (relative to the sample distribution) was associated with reduced incidence of CHD (adjusted hazard ratio [aHR] = 0.94 per standard deviation [SD] increase in LTL, [95% CI, 0.90 to 0.99], P = .010). Incidence of CHD was reduced by higher total volume of aPA (aHR = 0.82 per SD increase in LTL, [95% CI, 0.71 to 0.95], P = .010) but increased by higher total number of events (aHR = 1.11 per SD increase in LTL, [95% CI, 1.02 to 1.21], P = .020) after controlling for other aPA measures and covariates. However, none of the interactions between LTL and aPA measures was statistically significant (P = .171).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Telomeres are repetitive base pair sequences of TTAGGG at the end of chromosomes [1]. Critically short telomere length leads to permanent cell cycle arrest and cellular senescence [2]. Senescent cells secrete high levels of inflammatory cytokines, cell cycle regulators, growth factors, and tissue remodeling factors, known as the senescence-associated secretory phenotype (SASP) [3], which can exert deleterious effects on health outcomes, including cardiovascular disease [4]. Cellular senescence is one of the hallmarks of biological aging [5], and it can be a therapeutic target aiming to prevent or delay the onset of multiple diseases or conditions based on the geroscience hypothesis [6].
Whether physical activity is associated with telomere length remains inconclusive [7]. A systematic review and meta-analysis study [7] was conducted to evaluate the association between physical activity and telomere length. Most of the included studies measured telomere length in leukocytes using the quantitative polymerase chain reaction (qPCR) method. Different methods were used to measure physical activity, including questionnaires, physical activity monitors, and groups with different physical activity levels, e.g., athletes versus a control group. Partly due to the heterogeneity from measurements between studies, inconsistent findings on the association between physical activity and telomere length were reported: twenty studies did not find a statistically significant association, fifteen studies reported a positive association, while two studies described a U-shaped relationship.
It is unclear whether physical activity can be an intervention for telomere shortening [8]. Six randomized controlled trials were identified in the literature to examine the effect of physical activity on telomere shortening [8]. The meta-analysis results suggested that physical activity was not significantly associated with changes in telomere length. However, the estimates were uncertain as reflected in the wide confidence intervals. Furthermore, most of the included studies showed a moderate to high risk of bias from the randomization process or deviations from the protocol. Additionally, participants were predominantly obese or physically inactive so the findings may not be generalizable to the general population.
While it remains unclear if physical activity is associated with telomere length or telomere shortening that commonly occurs with aging, the benefits of physical activity for CHD is evident [9]. Being physically active reduces the incidence of CHD by 33–58% [9]. Results from a meta-analysis showed a dose–response relationship between physical activity and CHD [10]. The risk of CHD was reduced by 14%, comparing individuals who reported an equivalent of 150 min/week of moderate-intensity, leisure-time physical activity (LTPA) to those who reported no LTPA. More significantly, individuals who reported an equivalent of 300 min/week of moderate-intensity LTPA had a 20% lower risk of CHD than those who reported no LTPA. Similarly, the dose–response association was reported in a UK Biobank (UKB) study linking accelerometer measured physical activity to the incidence of cardiovascular disease [11].
The association of shorter LTL, relative to the sample distribution, with CHD is well documented in the literature [12] and the relationship is likely to be causal [13, 14]. Plausible mechanisms underlying the association between shorter LTL and CHD include oxidative stress [15], chronic inflammation [16], and endothelial cell senescence [17, 18], which may be reduced by physical activity [8, 19, 20]. We hypothesized that increased physical activity may mitigate the association between shorter LTL and CHD. To test the hypothesis, we conducted a prospective study using the UKB physical activity cohort with well-calibrated accelerometer data for at least 6.5 days (n = 54,180). This research is important as our findings will inform whether shorter telomere length can be targeted by physical activity to reduce the risk of incident CHD.
Methods
UK Biobank
Over 500,000 participants between ages 40 and 70 were recruited in the UKB between 2006 and 2010 [21]. Baseline assessments included detailed online questionnaires on health and lifestyle, physical measurements, and biological samples for future assays. Participants are followed up through linkages to electronic health records, including data from hospital admissions, primary care visits, cancer, and death registries. Subsets of participants returned to undergo additional assessments, e.g., multi-modality imaging scans and accelerometer-based physical activity (aPA) assessment [22].
Study design
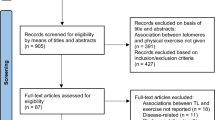
Baseline blood samples were used to measure LTL between 2006 and 2010. About 20% of the cohort participated in aPA assessment between 2013 and 2016. Since then, the included samples (detailed below) were followed up for incident coronary heart disease (CHD) via inpatient hospital episode statistics (HES) until death or the last censoring date depending on the country (9/30/2021 for England; 7/31/2021 for Scotland; 2/28/2018 for Wales) (Fig. 1).
Included samples
Among 502,445 active UKB participants, regardless of ancestry, 103,669 completed the aPA assessment. We excluded participants who had accelerometer data with poor calibration or quality, or had short wear time (first five items in the exclusion box of Fig. 2). We also excluded (1) participants diagnosed with CHD or any cancer (excluding non-melanoma skin cancers) prior to aPA assessment, (2) participants with any unusually large value of aPA measure (i.e., greater than the third quartile + 1.5 × interquartile range), and (3) participants with any missing value in CHD, LTL, aPA measures, or covariates. As a result, a total of 54,180 participants were included in analysis.
Data measurements
Leukocyte telomere length
DNA was extracted from peripheral blood leukocytes for all UKB participants at baseline (recruitment). Using a multiplex quantitative polymerase chain reaction (qPCR) technology, LTL referred to as the mean leukocyte telomere length was measured in T/S ratio, comparing the amount of the telomere amplification product (T) to that of a single-copy gene (S). LTL was adjusted for the influence of technical parameters by Codd et al. on behalf of the UKB [23]. Of those with LTL measurements at baseline in UKB (n = 488,400), 1884 participants had a second blood sample drawn between 2 and 10 years after the original sample, which was processed using the same methods and the LTL data were used to correct for regression dilution bias [23].
Disease diagnoses
Disease diagnoses (yes/no) were confirmed using baseline self-reported and inpatient HES data based on ICD-9 and ICD-10 codes. As described previously, we pre-excluded participants with any cancer (ICD-10 C00–C97; ICD-9 140–209), excluding non-melanoma skin cancers (ICD-10 C44; ICD-9 172). CHD cases were confirmed if any of the conditions were met: ICD-10 I20–I25; ICD-9 410–414. Time in years from aPA assessment to the first diagnosis of CHD was recorded for CHD cases and from aPA assessment to HES censoring date or date of death, whichever occurred first, for non-CHD controls.
Accelerometer-based physical activity
Participants attending the aPA assessment were asked to wear an Axivity AX3 triaxial accelerometer (https://axivity.com/product/ax3) on the wrist of their dominant hand, for 24 h a day for seven consecutive days [24]. There was no repeated aPA assessment at the time of manuscript submission. The acceleration was calibrated and summarized in x/y/z axes for each 1-s epoch using the Euclidean Norm Minus One (ENMO) [25]. We adopted an event-based approach to process aPA data. The event-based approach permits a robust analysis and quantification of patterns that facilitate physical activity research [26, 27]. A time series of “active” events was then extracted from the data. To identify the start and end of an “active” event, all values were capped at 40 milligravities (mg) to prevent skewing by outliers. The start of an event was defined as when the acceleration signal was equal to 40 mg and the end of the event was determined when the average acceleration for consecutive epochs fell below 32 mg (80% of 40 mg). This 80% criteria allowed for short periods when people may temporarily cease moving. Only events of at least 10 s were included in analysis to reduce the effect of wrist-specific/isolated movements on the data. Events were computed on a per-person and per-day basis. Each event was characterized by its start time, duration (seconds in each event), intensity (the mean value of acceleration in each event), and volume (the sum of accelerations in each event).
Five aPA measures were derived from the time series of events and the definitions are provided as follows.
-
1.
Total volume: total volume (mg) of all active events
-
2.
Total number of events: total number of active events
-
3.
Mean duration: mean duration (seconds) of all active events
-
4.
Mean intensity: mean of the mean intensity (mg/second) of all active events
-
5.
Peak intensity: 95th percentile of the 95th percentile acceleration values (mg/second) within active events, which tends to increase with longer events
Baseline covariates
Demographic data included age, self-reported sex (male or female), ethnicity (grouped into White, Black, South Asian, and Other), and education (from none to college or university degree, see Table 1), as well as baseline assessment center near participant’s residence. Socioeconomic status was measured by the Townsend deprivation index, an area-based measure of material deprivation, with higher scores representing higher levels of deprivation. Lifestyle factors included smoking status, alcohol intake frequency, and body mass index (BMI). Smoking status (never, former, or current) and alcohol intake frequency (never, special occasions only, one to three times a month, one or twice a week, three or four times a week, daily or almost daily) were assessed at baseline via online questionnaires.
Statistical methods
A descriptive analysis was conducted to summarize variables. The rank-based inverse normal transformation was applied to LTL and aPA measures among the included samples to normalize the data to zero mean and unit variance so the scales were unified before modeling. Following that, the Pearson correlation coefficients were calculated between aPA measures. The proportion of variance in total volume, attributed to other aPA measures, was estimated in a multivariable linear regression model.
Cox proportional hazards regression models were used to determine whether, and to what extent, LTL and aPA measures individually or all together were associated with incident CHD, adjusting for baseline covariates (age, sex, ethnicity, education, Townsend deprivation index, BMI, smoking status, alcohol intake frequency, and assessment center). In the survival analysis, time from aPA assessment to incident CHD was censored at the last HES follow-up date (9/30/2021 for England; 7/31/2021 for Scotland; 2/28/2018 for Wales) or date of death, whichever occurred first.
The non-linearity of LTL or aPA measures were evaluated using cubic penalized spline functions with 10 splines in the basis (default). In the joint analysis of LTL and all aPA measures, we evaluated the interactions between LTL and aPA measures in a forward manner, considering the significant aPA measures (P < 0.05) by the order of P-values in the Cox proportional hazards regression model with all aPA measures and covariates. P-values smaller than 5% were considered statistically significant. All the statistical analyses were performed in R 4.1.0.
Results
Table 1 shows characteristics of the included samples (n = 54,180) at baseline when LTL was measured. These participants were predominantly White (97.1%), female (57%), and well-educated (45% with a college or university degree). The mean age was 56.0 years (SD = 7.7) at baseline, with a mean follow-up of 5.7 years (SD = 1.1) to aPA assessment and 6.8 years (SD = 0.9) from aPA assessment to last HES update.
Participants tended to have better-than-average socioeconomic status and healthy lifestyles, with the mean Townsend deprivation index − 1.75 versus the population average of 0. The mean BMI was 26.7 (SD = 4.5). Fifty-eight percent of the samples were never smokers. Ninety-five percent of them drank, including 23% drinking daily or almost daily. The mean LTL (T/S ratio) for the whole sample was 0.8 (SD = 0.1) after adjusting for the influence of technical parameters.
During follow-up, 1986 incident CHD cases were identified, with the mean age at diagnosis 68.8 years (SD = 7.7). Of the CHD cases identified, 1505 cases were diagnosed between the aPA assessment and the start of COVID-19 pandemic (2/1/2020). The remaining cases (n = 481) were diagnosed after the pandemic started. Comparing the two periods, the incidence was similar, 528 cases per 100,000 person-years [before] versus 549 cases per 100,000 person-years [after], suggesting a minimal bias in CHD diagnosis due to COVID-19.
A descriptive summary of aPA measures, along with histograms to illustrate their distributions, is provided in Supplemental Table 1 and Supplemental Fig. 1. The Pearson correlations (r) between aPA measures after z-transformations were calculated (Supplemental Fig. 2). A higher mean intensity (r = 0.57) or peak intensity (r = 0.49) and a longer mean duration (r = 0.79) were correlated with a higher total volume. In contrast, a higher total number of events was correlated with a lower total volume (r = − 0.16). Eighty-seven percent of the variance in total volume was explained by other aPA measures (Supplemental Table 2). The multivariable linear regression analysis showed that all aPA measures were significantly and independently associated with total volume and, interestingly, that all associations were positive after controlling for other aPA measures (Supplemental Table 2). There was no significant collinearity based on variance inflation factors (VIF < 10) associated with each aPA measure (Supplemental Table 2).
Association between LTL and incident CHD
A scatterplot of incidence of CHD versus LTL showed an inverse linear relationship (Supplemental Fig. 3). Using Cox proportional hazards regression models, we showed a reduction of 18% in the risk of CHD per SD increase in LTL (unadjusted HR = 0.82, [95% CI, 0.79 to 0.86], P < 0.001). After adjusting for covariates, the association remained statistically significant, with a reduction of 6% in the risk of CHD per SD increase in LTL (adjusted HR [aHR] = 0.94, [95% CI, 0.90 to 0.99], P = 0.010). “Per SD” hereafter is referred to as per standard deviation increase in LTL or an aPA measure. Additionally, we tested the non-linearity of LTL association with incident CHD in the adjusted Cox regression model and the result was not statistically significant (P = 0.131).
Associations between and aPA measures and incident CHD
The relationship between each aPA measure and incidence of CHD, as shown in Supplemental Fig. 4, was approximately linear. When they were modeled jointly in a multivariable Cox proportional hazards regression model, allowing for non-linear relationships with incident CHD, mean intensity was the only one showing significant non-linearity (P = 0.016). In the adjusted Cox regression model with a cubic penalized spline function (non-linear function) of mean intensity and other aPA measures assuming linear relationships, total volume and total number of events were the only two statistically significant aPA measures. For every 1-SD increase in total volume of aPA, the risk of incident CHD was reduced by 18% (aHR = 0.82 per SD, [95% CI, 0.71 to 0.95], P = 0.010), and the risk of incident CHD was increased by 11% per SD increase in the number of events (or aHR = 1.11 per SD, [95% CI, 1.02 to 1.21], P = 0.020) when other aPA measures and covariates are controlled. Of note, mean intensity modeled via a cubic penalized spline function was not significantly associated with incident CHD (P = 0.451) after adjusting for covariates (Fig. 3). The HRs comparing a mean intensity z-score to the mean of mean intensity z-scores (= 0) are selectively included in Fig. 3.
Associations between accelerometer-based physical activity (aPA) measures and incident coronary heart disease (CHD) risk in the Cox regression model for incident CHD on the aPA measures of total volume, total number of events, mean duration, and peak intensity, assuming linear relationships, and mean intensity via a cubic penalized spline function (non-linear function to account for significant non-linearity), without and with adjustment for the baseline covariates of age, sex, ethnicity, Townsend deprivation index, smoking status, alcohol intake frequency, and assessment center
Interactions between TL and aPA measures on incident CHD
For visualization, we categorized each aPA measure into low, medium, and high, corresponding to the quartile groups 0–25% (low), 25–75% (moderate), and 75–100% (high). As shown in Supplemental Fig. 5, longer LTL was associated with reduced incidence of CHD, and the three aPA groups showed similar slopes.
In the association analysis between aPA measures (including a non-linear function of mean intensity) and incident CHD, total volume (P = 0.010) and total number of events (P = 0.020) were the only two aPA measures that reached the significance level of 5% after adjusting for covariates and other aPA measures. Given the main effects of LTL and all aPA measures plus covariates, we first included the interaction term between LTL and total volume and the interaction P-value was not statistically significant (standardized β = 0.011, [95% CI − 0.033 to 0.055], P = 0.626) so the interaction term was not included in the model. Next, we included the interaction term between LTL and total number of events and the interaction term was not statistically significant either (standardized β = 0.005, [95% CI, − 0.039 to 0.049], P = 0.831). We also ran an overall test for any significant interactions between LTL and aPA measures by comparing the model with the main effects of LTL, all aPA measures, and covariates versus that with additional terms for interactions between LTL and individual aPA measures. Consistently, the result was not statistically significant (P = 0.171), which indicated that none of aPA measures significantly modified the risk of CHD associated with shorter LTL.
Independent associations of LTL and aPA measures with incident CHD
There was a lack of evidence that aPA measures moderate the association between LTL and incident CHD. LTL and aPA measures were independently associated with incidence of CHD, which was suggested by minimally changed aHRs associated with LTL and aPA measures (absolute differences smaller than 0.01), comparing the model with both LTL and aPA measures and the models with either LTL or aPA measures (Fig. 4). Additionally, total volume of aPA was most strongly associated with incident CHD after adjusting for other aPA measures. We also did not find a significant association between LTL and total volume (dependent variable) after adjusting for other aPA and covariates measures (standardized β associated with LTL 0.0008, [95% CI, − 0.0022 to 0.0038], P = 0.600) or covariates only (standardized β associated with LTL 0.0015, [95% CI, − 0.0065 to 0.0095], P = 0.710).
Joint model of leukocyte telomere length (LTL) and accelerometer-based physical activity (aPA) measures (total volume, total number of events, mean duration, and peak intensity assuming linear relationships and mean intensity via a cubic penalized spline function to account for significant non-linearity) versus models with LTL or aPA measures only. aLTL only: CHD ~ LTL + age + sex + ethnicity + education + Townsend deprivation index + smoking status + alcohol intake frequency + baseline assessment center; aPAs only: CHD ~ aPA measures (total volume, total number of events, mean duration, and peak intensity assuming linear relationships and mean intensity via a cubic penalized spline function to account for significant non-linearity) + age + sex + ethnicity + education + Townsend deprivation index + smoking status + alcohol intake frequency + baseline assessment center. bLTL and aPAs: CHD ~ LTL + aPA measures (total volume, total number of events, mean duration, and peak intensity assuming linear relationships and mean intensity via a cubic penalized spline function to account for significant non-linearity) + age + sex + ethnicity + education + Townsend deprivation index + smoking status + alcohol intake frequency + baseline assessment center
Discussion
To our knowledge, this is the first large population-based study to evaluate the interaction of LTL with aPA measures on the risk of incident CHD. Longer telomere length was significantly associated with reduced risk of CHD. Likewise, higher total volume and lower total number of events of aPA were also significantly associated with reduced incidence of CHD. However, none of aPA measures showed a significant moderation effect on shorter LTL and incident CHD. Our results also suggested that LTL and aPA measures are independently associated with incident CHD.
The non-significant finding on the moderation of aPA measures is unlikely due to inadequate statistical power, as the 95% confidence interval for the standardized β associated with the interaction between LTL and an aPA measure is extremely short and centered around the null value zero. For example, when considering the interaction between LTL and total volume in the model with the main effects of LTL, aPA measures, and covariates, the standardized β and 95% confidence interval for the interaction was 0.011 ([95% CI, − 0.033 to 0.055], P = 0.626), and that when considering the interaction between LTL and total number of events was 0.005 ([95% CI, − 0.039 to 0.049], P = 0.831).
We have shown that higher total volume and lower number of events of aPA were significantly associated with decreased risk of CHD when modeled simultaneously with other aPA measures and adjusting for covariates (Fig. 3). For any given higher total volume, with fewer events, either the mean duration or the mean intensity must be higher compared to the same volume with more events. While it might be expected that lower mean intensity would be associated with a higher risk of CHD, in this multivariable model, it was associated with a lower risk of CHD for the following reasons. To achieve a higher total volume of aPA with the same number of events, a lower mean intensity must be accompanied by a longer mean duration and the duration offers the protection. By the same token, to achieve a higher total volume of aPA with the same number of events, a shorter mean duration must co-exist with a higher mean intensity, explaining why higher mean duration, in the multivariable model, appears to reduce the risk of CHD—albeit the associations with mean intensity and mean duration were not statistically significant (Fig. 3).
While several mechanisms have been postulated [28], a widely accepted hypothesis is that shorter telomeres lead to CHD through major SASP factors in different senescent cell types that contribute to atherosclerosis, e.g., IL-6 (forming atherosclerotic plagues and causing thrombosis), TNF (recruiting more immune cells and forming atherosclerotic plaques), and VEGF (promoting plaque angiogenesis and vascular remodeling) in senescent endothelial cells [29]. Physical activity may lower protein levels of SASP factors, which reduces the risk of CHD. While we found that none of the aPA measures significantly moderated the association of shorter LTL with incident CHD, a preclinical study showed that exercise decreased the protein expression of p53 and p16 (senescence markers) in endothelial cells from aortic lysates in young mice [17]. In a human study, the protein expression of p53, p21, and p16 (senescence markers) in endothelial cells from antecubital veins were significantly higher in older sedentary adults than in young sedentary adults, but the age-related differences were not present between older exercising adults and young sedentary adults [20]. Additionally, a recent study [30] showed that a 12-week structured exercise program significantly lowered the levels of several senescence-related proteins in peripheral blood CD3 + T cells, including p16, p21, cGAS, TNFα, and PD1, which may subsequently reduce the risk of incident CHD. Whether the improvement is universal or only applied to certain senescent cell types and tissues requires further investigation. A comprehensive study that accounts for the heterogeneity in major SASP factors in different senescent cell types and tissues is warranted to understand the relationships among short telomere length, physical activity, and senescence to CHD.
Several limitations need to be considered when interpreting the results. First, telomeres from cardiac tissues may be more relevant to this study than peripheral blood leukocytes, but the correlations between telomere length among different tissues are generally positive [31]. Second, LTL was only measured once at recruitment; therefore, we could not relate changes in LTL due to physical activity to the risk of CHD. Third, due to the unprecedentedly large sample size, the qPCR method was chosen for feasibility reasons but introduced higher variability than other techniques. To overcome the challenge, Codd et al. [23], on behalf of the UKB, removed the technical differences using statistical methods and the adjusted measurements warranted high intra-assay reliability and reproducibility based on the quality control evaluation. Fourth, although multiple measures are desired to assess different domains of physical activity, the 7-day aPA measures remain limited in modeling the temporal profiles of acceleration. Fifth, in addition to physical activity, other potential moderators such as demographics (e.g., age, sex, and ethnicity), lifestyle factors (e.g., smoking and alcohol intake), and disease status were not considered and the study samples of this present study were dominated by participants of European descent, with the mean age 56.0 years at aPA assessment. Likely, the moderation effect of physical activity may exist in subpopulations, which will require future investigation. Sixth, the study period includes the COVID-19 pandemic, which likely changed healthcare-seeking behaviors, but we found a similar incidence of CHD before and after the pandemic. Lastly, UKB participants are healthier than the general population, which may reduce exposure-outcome associations, but the effect is minimal due to significant heterogeneity in exposures [32].
In conclusion, longer duration physical activity events with a desired total volume are most beneficial for CHD, regardless of intensity. Physical activity, however, does not play a moderating role in the association between shorter LTL and incident CHD. Additional interventions are needed to reduce the adverse effects of shorter LTL on the risk of CHD.
Data availability
Data used in this project are available via application to UK Biobank.
References
Blackburn EH, Epel E. The telomere effect: a revolutionary approach to living younger, healthier, longer. 1st ed. New York: Grand Central Publishing; 2017.
Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25(3):585–621.
Basisty N, Kale A, Jeon OH, Kuehnemann C, Payne T, Rao C, Holtz A, Shah S, Sharma V, Ferrucci L, Campisi J, Schilling B. A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol. 2020;18(1):e3000599.
Mehdizadeh M, Aguilar M, Thorin E, Ferbeyre G, Nattel S. The role of cellular senescence in cardiac disease: basic biology and clinical relevance. Nat Rev Cardiol. 2022;19(4):250–64.
López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–217.
Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, Franceschi C, Lithgow GJ, Morimoto RI, Pessin JE, Rando TA, Richardson A, Schadt EE, Wyss-Coray T, Sierra F. Geroscience: linking aging to chronic disease. Cell. 2014;159(4):709–13.
Mundstock E, Zatti H, Louzada FM, Oliveira SG, Guma FTCR, Paris MM, Rueda AB, Machado DG, Stein RT, Jones MH, Sarria EE, Barbé-Tuana FM, Mattiello R. Effects of physical activity in telomere length: systematic review and meta-analysis. Ageing Res Rev. 2015;22:72–80.
Valente C, Andrade R, Alvarez L, Rebelo-Marques A, Stamatakis E, Espregueira-Mendes J. Effect of physical activity and exercise on telomere length: systematic review with meta-analysis. J Am Geriatr Soc. 2021;69(11):3285–300.
Powell KE, Thompson PD, Caspersen CJ, Kendrick JS. Physical activity and the incidence of coronary heart disease. Annu Rev Public Health. 1987;8:253–87.
Sattelmair J, Pertman J, Ding EL, Kohl HW, Haskell W, Lee IM. Dose response between physical activity and risk of coronary heart disease: a meta-analysis. Circulation. 2011;124(7):789–95.
Ramakrishnan R, Doherty A, Smith-Byrne K, Rahimi K, Bennett D, Woodward M, Walmsley R, Dwyer T. Accelerometer measured physical activity and the incidence of cardiovascular disease: evidence from the UK Biobank cohort study. Paluch A, editor. PLoS Med. 2021;18(1):e1003487.
Haycock PC, Heydon EE, Kaptoge S, Butterworth AS, Thompson A, Willeit P. Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. BMJ. 2014;8(349):g4227.
Telomeres Mendelian Randomization Collaboration, Haycock PC, Burgess S, Nounu A, Zheng J, Okoli GN, Bowden J, Wade KH, Timpson NJ, Evans DM, Willeit P, Aviv A, Gaunt TR, Hemani G, Mangino M, Ellis HP, Kurian KM, Pooley KA, Eeles RA, Lee JE, Fang S, Chen WV, Law MH, Bowdler LM, Iles MM, Yang Q, Worrall BB, Markus HS, Hung RJ, Amos CI, Spurdle AB, Thompson DJ, O’Mara TA, Wolpin B, Amundadottir L, Stolzenberg-Solomon R, Trichopoulou A, Onland-Moret NC, Lund E, Duell EJ, Canzian F, Severi G, Overvad K, Gunter MJ, Tumino R, Svenson U, van Rij A, Baas AF, Bown MJ, Samani NJ, van t’Hof FNG, Tromp G, Jones GT, Kuivaniemi H, Elmore JR, Johansson M, Mckay J, Scelo G, Carreras-Torres R, Gaborieau V, Brennan P, Bracci PM, Neale RE, Olson SH, Gallinger S, Li D, Petersen GM, Risch HA, Klein AP, Han J, Abnet CC, Freedman ND, Taylor PR, Maris JM, Aben KK, Kiemeney LA, Vermeulen SH, Wiencke JK, Walsh KM, Wrensch M, Rice T, Turnbull C, Litchfield K, Paternoster L, Standl M, Abecasis GR, SanGiovanni JP, Li Y, Mijatovic V, Sapkota Y, Low SK, Zondervan KT, Montgomery GW, Nyholt DR, van Heel DA, Hunt K, Arking DE, Ashar FN, Sotoodehnia N, Woo D, Rosand J, Comeau ME, Brown WM, Silverman EK, Hokanson JE, Cho MH, Hui J, Ferreira MA, Thompson PJ, Morrison AC, Felix JF, Smith NL, Christiano AM, Petukhova L, Betz RC, Fan X, Zhang X, Zhu C, Langefeld CD, Thompson SD, Wang F, Lin X, Schwartz DA, Fingerlin T, Rotter JI, Cotch MF, Jensen RA, Munz M, Dommisch H, Schaefer AS, Han F, Ollila HM, Hillary RP, Albagha O, Ralston SH, Zeng C, Zheng W, Shu XO, Reis A, Uebe S, Hüffmeier U, Kawamura Y, Otowa T, Sasaki T, Hibberd ML, Davila S, Xie G, Siminovitch K, Bei JX, Zeng YX, Försti A, Chen B, Landi S, Franke A, Fischer A, Ellinghaus D, Flores C, Noth I, Ma SF, Foo JN, Liu J, Kim JW, Cox DG, Delattre O, Mirabeau O, Skibola CF, Tang CS, Garcia-Barcelo M, Chang KP, Su WH, Chang YS, Martin NG, Gordon S, Wade TD, Lee C, Kubo M, Cha PC, Nakamura Y, Levy D, Kimura M, Hwang SJ, Hunt S, Spector T, Soranzo N, Manichaikul AW, Barr RG, Kahali B, Speliotes E, Yerges-Armstrong LM, Cheng CY, Jonas JB, Wong TY, Fogh I, Lin K, Powell JF, Rice K, Relton CL, Martin RM, Davey Smith G. Association between telomere length and risk of cancer and non-neoplastic diseases: a Mendelian randomization study. JAMA Oncol. 2017;3(5):636–651.
Kuo C, Pilling LC, Kuchel GA, Ferrucci L, Melzer D. Telomere length and aging‐related outcomes in humans: a Mendelian randomization study in 261,000 older participants. Aging Cell [Internet]. 2019 Dec [cited 2022 Sep 4];18(6). Available from: https://onlinelibrary.wiley.com/doi/10.1111/acel.13017
Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci USA. 2004;101(49):17312–5.
O’Donovan A, Pantell MS, Puterman E, Dhabhar FS, Blackburn EH, Yaffe K, Cawthon RM, Opresko PL, Hsueh WC, Satterfield S, Newman AB, Ayonayon HN, Rubin SM, Harris TB, Epel ES, Health Aging and Body Composition Study. Cumulative inflammatory load is associated with short leukocyte telomere length in the Health, Aging and Body Composition Study. PLoS One. 2011;6(5):e19687.
Minamino T, Miyauchi H, Yoshida T, Ishida Y, Yoshida H, Komuro I. Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation. 2002;105(13):1541–4.
Matthews C, Gorenne I, Scott S, Figg N, Kirkpatrick P, Ritchie A, Goddard M, Bennett M. Vascular smooth muscle cells undergo telomere-based senescence in human atherosclerosis: effects of telomerase and oxidative stress. Circ Res. 2006;99(2):156–64.
Werner C, Fürster T, Widmann T, Pöss J, Roggia C, Hanhoun M, Scharhag J, Büchner N, Meyer T, Kindermann W, Haendeler J, Böhm M, Laufs U. Physical exercise prevents cellular senescence in circulating leukocytes and in the vessel wall. Circulation. 2009;120(24):2438–47.
Rossman MJ, Kaplon RE, Hill SD, McNamara MN, Santos-Parker JR, Pierce GL, Seals DR, Donato AJ. Endothelial cell senescence with aging in healthy humans: prevention by habitual exercise and relation to vascular endothelial function. Am J Physiol-Heart Circ Physiol. 2017;313(5):H890–5.
Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, Downey P, Elliott P, Green J, Landray M, Liu B, Matthews P, Ong G, Pell J, Silman A, Young A, Sprosen T, Peakman T, Collins R. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779.
Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, Motyer A, Vukcevic D, Delaneau O, O’Connell J, Cortes A, Welsh S, Young A, Effingham M, McVean G, Leslie S, Allen N, Donnelly P, Marchini J. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562(7726):203–9.
Codd V, Denniff M, Swinfield C, Warner SC, Papakonstantinou M, Sheth S, Nanus DE, Budgeon CA, Musicha C, Bountziouka V, Wang Q, Bramley R, Allara E, Kaptoge S, Stoma S, Jiang T, Butterworth AS, Wood AM, Di Angelantonio E, Thompson JR, Danesh JN, Nelson CP, Samani NJ. Measurement and initial characterization of leukocyte telomere length in 474,074 participants in UK Biobank. Nat Aging. 2022;2(2):170–9.
Doherty A, Jackson D, Hammerla N, Plötz T, Olivier P, Granat MH, White T, van Hees VT, Trenell MI, Owen CG, Preece SJ, Gillions R, Sheard S, Peakman T, Brage S, Wareham NJ. Large scale population assessment of physical activity using wrist worn accelerometers: the UK Biobank study. Buchowski M, editor. PLoS One. 2017;12(2):e0169649.
van Hees VT, Fang Z, Langford J, Assah F, Mohammad A, da Silva ICM, Trenell MI, White T, Wareham NJ, Brage S. Autocalibration of accelerometer data for free-living physical activity assessment using local gravity and temperature: an evaluation on four continents. J Appl Physiol (1985). 2014;117(7):738–44.
Granat MH. Event-based analysis of free-living behaviour. Physiol Meas. 2012;33(11):1785–800.
Granat M, Clarke C, Holdsworth R, Stansfield B, Dall P. Quantifying the cadence of free-living walking using event-based analysis. Gait Posture. 2015;42(1):85–90.
Zhan Y, Hägg S. Telomere length and cardiovascular disease risk. Curr Opin Cardiol. 2019;34(3):270–4.
Sun Y, Wang X, Liu T, Zhu X, Pan X. The multifaceted role of the SASP in atherosclerosis: from mechanisms to therapeutic opportunities. Cell Biosci. 2022;12(1):74.
Englund DA, Sakamoto AE, Fritsche CM, Heeren AA, Zhang X, Kotajarvi BR, Lecy DR, Yousefzadeh MJ, Schafer MJ, White TA, Atkinson EJ, LeBrasseur NK. Exercise reduces circulating biomarkers of cellular senescence in humans. Aging Cell. 2021;20(7):e13415.
Demanelis K, Jasmine F, Chen LS, Chernoff M, Tong L, Delgado D, Zhang C, Shinkle J, Sabarinathan M, Lin H, Ramirez E, Oliva M, Kim-Hellmuth S, Stranger BE, Lai TP, Aviv A, Ardlie KG, Aguet F, Ahsan H, GTEx Consortium, Doherty JA, Kibriya MG, Pierce BL. Determinants of telomere length across human tissues. Science. 2020;369(6509):eaaz6876.
Fry A, Littlejohns TJ, Sudlow C, Doherty N, Adamska L, Sprosen T, Collins R, Allen NE. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am J Epidemiol. 2017;186(9):1026–34.
Acknowledgements
The authors would like to acknowledge the use of the University of Exeter High Performance Computing (HPC) facility in carrying out this work. Lastly, we thank Joshua Twaites for sharing the code to identify aPA events.
Funding
This research was funded by the National Institute of Nursing Research, National Institutes of Health, USA (R21NR018963-01A1). CLK, GAK, MH, LCP, DM, and MX were partly supported by the grant above. CLK and GAK were partly supported by P30AG067988. Data access to UK Biobank was granted under application no. 14631 (Melzer 2015). This research used data assets made available by National Safe Haven as part of the Data and Connectivity National Core Study, led by Health Data Research UK in partnership with the Office for National Statistics and funded by UK Research and Innovation (research which commenced between 1st October 2020 and 31st March 2021 grant ref MC_PC_20029; 1st April 2021 -30th September 2022 grant ref MC_PC_20058). This research also used data provided by patients and collected by the NHS as part of their care and support. Copyright © (year), NHS England. Re-used with the permission of the NHS England [and/or UK Biobank]. All rights reserved.
Author information
Authors and Affiliations
Contributions
CLK, MH, BM, LCP, and DM designed the study; MX and CLK performed statistical analyses; ARW, MH, BM, and LCP processed raw accelerometer data; BSD, MH, and CLK drafted the initial manuscript, and all the authors reviewed the manuscript. The authors were not precluded from accessing data in the study and they accepted responsibility to submit for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Xiang, M., Pilling, L.C., Melzer, D. et al. Does physical activity moderate the association between shorter leukocyte telomere length and incident coronary heart disease? Data from 54,180 UK Biobank participants. GeroScience 46, 1331–1342 (2024). https://doi.org/10.1007/s11357-023-00890-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-023-00890-7