Abstract
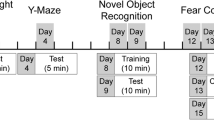
Hypogonadism is a common comorbidity associated with HIV-1 that is more prevalent among infected individuals over the age of 45. The underlying mechanisms are unknown, but both combined antiretroviral therapeutics and HIV-1 proteins, such as trans-activator of transcription protein (Tat), dysregulate steroid-synthetic mechanisms including lipid storage/synthesis and mitochondrial function. Thus, Tat expression may accelerate age-related comorbidities partly by impairing endocrine function. Few studies exist of Tat-mediated behavioral deficits in aged animals and effects of endocrine status have not been investigated. Accordingly, we tested whether conditional Tat expression in aged (~ 1.5 years old), female, Tat-transgenic [Tat(+)] mice increases anxiety-like behavior, impairs cognition, and augments mechanical allodynia, when compared to age-matched controls that do not express Tat protein [Tat(−)]. We further tested whether aged mice that maintained their endocrine status (pre-estropausal) were more resilient to Tat/age-related comorbidities than peri- or post-estropausal mice. Tat and endocrine aging status exerted separate and interacting effects that influenced anxiety-like and cognitive behaviors. Peri- and post-estropausal mice exhibited greater anxiety-like behavior in the elevated plus-maze and impaired learning in the radial arm water maze compared to pre-estropausal mice. Irrespective of estropause status, Tat(+) mice demonstrated impaired learning, reduced grip strength, and mechanical allodynia compared to Tat(−) mice. Tat exposure reduced circulating estradiol in post-estropausal mice and increased the estradiol-to-testosterone ratio in pre-estropausal mice. Changes in circulating estradiol, testosterone, and progesterone correlated with grip strength. Thus, endocrine status is an important factor in age-related anxiety, cognition, neuromuscular function, and allodynia that can be accelerated by HIV-1 Tat protein.







Similar content being viewed by others
References
Thakur KT, Boubour A, Saylor D, Das M, Bearden DR, Birbeck GL. Global HIV neurology: a comprehensive review. AIDS. 2019;33:163–84.
Veenhuis RT, Clements JE, Gama L. HIV eradication strategies: implications for the central nervous system. Curr HIV/AIDS Rep. 2019;16:96–104.
Saylor D, Dickens AM, Sacktor N, Haughey N, Slusher B, Pletnikov M, et al. HIV-associated neurocognitive disorder — pathogenesis and prospects for treatment. Nat Rev Neurol. 2016;12:234–48.
Cañizares S, Cherner M. Ellis and RJ. HIV and aging: effects on the central nervous system. Semin Neurol. 2014;34:27–34.
Cohen RA, Seider TR, Navia B. HIV effects on age-associated neurocognitive dysfunction: premature cognitive aging or neurodegenerative disease? Alzheimers Res Ther. 2015;7:37.
Bing EG, Burnam MA, Longshore D, Fleishman JA, Sherbourne CD, London AS, et al. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch Gen Psychiatry. 2001;58:721–8.
Woods SP, Moore DJ, Weber E, Grant I. Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychol Rev. 2009;19:152–68.
Mann R, Sadosky A, Schaefer C, Baik R, Parsons B, Nieshoff E, et al. Burden of HIV-related neuropathic pain in the United States. J Int Assoc Provid AIDS Care. 2016;15:114–25.
Aziz-Donnelly A, Harrison TB. Update of HIV-associated sensory neuropathies. Curr Treat Options Neurol. 2017;19:336–40.
Stauch KL, Emanuel K, Lamberty BG, Morsey B, Fox HS. Central nervous system-penetrating antiretrovirals impair energetic reserve in striatal nerve terminals. J Neuro-Oncol. 2017;23:795–807.
Hu G, Liao K, Yang L, Pendyala G, Kook Y, Fox HS, et al. Tat-mediated induction of miRs-34a & -138 promotes astrocytic activation via downregulation of SIRT1: implications for aging in HAND. J NeuroImmune Pharmacol. 2017;12:420–32.
Centers for Disease Control and Prevention. HIV Surveillance Report, 2018 (Preliminary); vol. 30. http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. 2018.
Clark RA, Cohn SE, Jarek C, Craven KS, Lyons C, Jacobson M, et al. Perimenopausal symptomatology among HIV-infected women at least 40 years of age. J Acquir Immune Defic Syndr. 2000;23:99–100.
Schoenbaum EE, Hartel D, Lo Y, Howard AA, Floris-Moore M, Arnsten JH, et al. HIV infection, drug use, and onset of natural menopause. Clin Infect Dis. 2005;41:1517–24.
Ferreira CE, Pinto-Neto AM, Conde DM, Costa-Paiva L, Morais SS, Magalhães J. Menopause symptoms in women infected with HIV: prevalence and associated factors. Gynecol Endocrinol. 2007;23:198–205.
Fan MD, Maslow BS, Santoro N, Schoenbaum E. HIV and the menopause. Menopause Int. 2008;14:163–8.
Imai K, Sutton MY, Mdodo R, del Rio C. HIV and menopause: a systematic review of the effects of HIV infection on age at menopause and the effects of menopause on response to antiretroviral therapy. Obs Gynecol Int. 2013;2013:340309.
Scherzer R, Bacchetti P, Messerlian G, Goderre J, Maki PM, Seifer DB, et al. Impact of CD4+ lymphocytes and HIV infection on antimüllerian hormone levels in a large cohort of HIV-infected and -uninfected women. Am J Reprod Immunol. 2015;73:273–84.
Scherzer R, Greenblatt RM, Merhi ZO, Kassaye S, Messerlian GL, Maki PM, et al. Use of antimüllerian hormone to predict the menopausal transition in HIV-infected women. Am J Obstet Gynecol. 2017;216:46.e1–46.e11.
Slama L, Jacobson LP, Li X, Jr FJP, Margolick JB, Kingsley LA, et al. Longitudinal changes over 10 years in free testosterone among HIV-infected and HIV-uninfected men. J Acquir Immune Defic Syndr. 2016;71:57–64.
Cejtin HE, Evans CT, Greenblatt R, Minkoff H, Weber KM, Wright R, et al. Prolonged amenorrhea and resumption of menses in women with HIV. J Women’s Heal. 2018;27:1441–8.
Schnall R, Jia H, Olender S, Gradilla M, Reame N. In people living with HIV (PLWH), menopause (natural or surgical) contributes to the greater symptom burden in women: results from an online US survey. Menopause. 2018;25:744–52.
Karim R, Mack WJ, Kono N, Tien PC, Anastos K, Lazar J, et al. Gonadotropin and sex steroid levels in HIV-infected premenopausal women and their association with subclinical atherosclerosis in HIV-infected and -uninfected women in the women's interagency HIV study (WIHS). J Clin Endocrinol Metab. 2013;98:E610–8.
Tariq S, Anderson J, Burns F, Delpech V, Gilson R, Sabin C. The menopause transition in women living with HIV: current evidence and future avenues of research. J Virus Erad. 2016;2:114–6.
Valcour V, Shikuma C, Shiramizu B, Watters M, Poff P, Selnes OA, et al. Higher frequency of dementia in older HIV-1 individuals: the Hawaii Aging with HIV-1 Cohort. Neurology. 2004;63:822–7.
Valcour V, Shikuma C, Shiramizu B, Watters M, Poff P, Selnes OA, et al. Age, apolipoprotein E4, and the risk of HIV dementia: the Hawaii Aging with HIV Cohort. J Neuroimmunol. 2004;157:197–202.
Clifford KM, Samboju V, Cobigo Y, Milanini B, Marx GA, Hellmuth JM, et al. Progressive brain atrophy despite persistent viral suppression in HIV over age 60. J Acquir Immune Defic Syndr. 2017;76:289–97.
Milanini B, Catella S, Perkovich B, Esmaeili-firidouni P, Wendelken L, Paul R, et al. Psychiatric symptom burden in older people living with HIV with and without cognitive impairment: the UCSF HIV over 60 cohort study. AIDS Care. 2017;29:1178–85.
Rubtsova AA, Marquine MJ, Depp C, Holstad M, Ellis RJ, Letendre S, et al. Psychosocial correlates of frailty among HIV-infected and HIV-uninfected adults. J Behav Med. 2019;45:210–20.
Blanco JR, Barrio I, Ramalle-Gómara E, Beltran MI, Ibarra V, Metola L, et al. Gender differences for frailty in HIV-infected patients on stable antiretroviral therapy and with an undetectable viral load. PLoS One. 2019;14:e0215764.
Ryan AS, Roy A, Oursler KK. Gait and balance biomechanics in older adults with and without human immunodeficiency virus. AIDS Res Hum Retrovir. 2019;35:1089–94.
Weinberg A, Enomoto L, Marcus R, Canniff J. Effect of menstrual cycle variation in female sex hormones on cellular immunity and regulatio. J Reprod Immunol. 2011;89:70–7.
Re MC, Furlini G, Vignoli M, Ramazzotti E, Roderigo G, Rosa VD, et al. Effect of antibody to HIV-1 Tat protein on viral replication in vitro and progression of HIV-1 disease in vivo. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;10:408–16.
Hudson L, Liu J, Nath A, Jones M, Raghavan R, Narayan O, et al. Detection of the human immunodeficiency virus regulatory protein tat in CNS tissues. J Neuro-Oncol. 2000;6:145–55.
Haughey NJ, Nath A, Mattson MP, Slevin JT, Geiger JD. HIV-1 tat through phosphorylation of NMDA receptors potentiates glutamate excitotoxicity. J Neurochem. 2001;78:457–67.
King JE, Eugenin EA, Buckner CM, Berman JW. HIV tat and neurotoxicity. Microbes Infect. 2006;8:1347–57.
Norman JP, Perry SW, Kasischke KA, David J. Volsky, Gelbard HA. HIV-1 trans activator of transcription protein elicits mitochondrial hyperpolarization and respiratory deficit, with dysregulation of complex IV and nicotinamide adenine dinucleotide homeostasis in cortical neurons. J Immunol 2007;178:869–876.
Fitting S, Ignatowska-Jankowska BM, Bull C, Skoff RP, Lichtman AH, Wise LE, et al. Synaptic dysfunction in the hippocampus accompanies learning and memory deficits in human immunodeficiency virus type-1 tat transgenic mice. Biol Psychiatry. 2013;73:443–53.
Fitting S, Stevens DL, Khan FA, Scoggins KL, Enga RM, Beardsley PM, et al. Morphine tolerance and physical dependence are altered in conditional HIV-1 tat transgenic mice. J Pharmacol Exp Ther. 2016;356:96–105.
Fitting S, Xu R, Bull C, Buch SK, El-Hage N, Nath A, et al. Interactive comorbidity between opioid drug abuse and HIV-1 Tat: chronic exposure augments spine loss and sublethal dendritic pathology in striatal neurons. Am J Pathol. 2010;177:1397–410.
Gonek M, McLane VD, Stevens DL, Lippold K, Akbarali HI, Knapp PE, et al. CCR5 mediates HIV-1 Tat-induced neuroinflammation and influences morphine tolerance, dependence, and reward. Brain Behav Immun. 2018;69:124–38.
Ensoli B, Fiorelli V, Ensoli F, Cafaro A, Titti F, Buttò S, et al. Candidate HIV-1 Tat vaccine development: from basic science to clinical trials. AIDS. 2006;20:2245–61.
Johnson TP, Patel K, Johnson KR, Maric D, Calabresi PA, Hasbun R, et al. Induction of IL-17 and nonclassical T-cell activation by HIV-Tat protein. Proc Natl Acad Sci U S A. 2013;110:13588–93.
Henderson LJ, Johnson TP, Smith BR, Reoma LB, Santamaria UA, Bachani M, et al. Presence of Tat and transactivation response element in spinal fluid despite antiretroviral therapy. AIDS. 2019;33:S145–57.
Mediouni S, Darque A, Baillat G, Ravaux I, Dhiver C, Tissot-Dupont H, et al. Antiretroviral therapy does not block the secretion of the human immunodeficiency virus Tat protein. Infect Disord Drug Targets. 2012;12:81–6.
Rochira V, Zirilli L, Orlando G, Santi D, Brigante G, Diazzi C, et al. Premature decline of serum total testosterone in HIV-infected men in the HAART-era. PLoS One. 2011;6:e28512.
Rochira V, Guaraldi G. Hypogonadism in the HIV-infected man. Endocrinol Metab Clin N Am. 2014;43:709–30.
Maritz MF, Ray RM, Bick AJ, Tomasicchio M, Woodland JG, Govender Y, et al. Medroxyprogesterone acetate, unlike norethisterone, increases HIV-1 replication in human peripheral blood mononuclear cells and an indicator cell line, via mechanisms involving the glucocorticoid receptor, increased CD4/CD8 ratios and CCR5 levels. PLoS One. 2018;13(4):e0196043.
Ferri KF, Jacotot E, Blanco J, Este JA, Kroemer G. Mitochondrial control of cell death induced by HIV-1-encoded proteins. Ann N Y Acad Sci. 2000;926:149–64.
Rozzi SJ, Avdoshina V, Fields JA, Trejo M, Ton HT, Ahern GP, et al. Human immunodeficiency virus promotes mitochondrial toxicity. Neurotox Res. 2017;32:723–33.
Fields JA, Ellis RJ. HIV in the cART era and the mitochondrial: immune interface in the CNS. Int Rev Neurobiol. 2019;145:29–65.
Cotto B, Natarajaseenivasan K, Ferrero K, Wesley L, Sayre M, Langford D. Disorders in cocaine user patients. Glia. 2018;66:889–902.
Mohseni Ahooyi T, Shekarabi M, Torkzaban B, Langford TD, Burdo TH, Gordon J, et al. Dysregulation of neuronal cholesterol homeostasis upon exposure to HIV-1 Tat and cocaine revealed by RNA-sequencing. Sci Rep. 2018;8:16300.
Kendall SL, Anderson CF, Nath A, Turchan-Cholewo J, Land CL, Mactutus CF, et al. Gonadal steroids differentially modulate neurotoxicity of HIV and cocaine: testosterone and ICI 182,780 sensitive mechanism. BMC Neurosci. 2005;6:40.
Heron PM, Turchan-Cholewo J, Bruce-Keller AJ, Wilson ME. Estrogen receptor alpha inhibits the estrogen-mediated suppression of HIV transcription in astrocytes: implications for estrogen neuroprotection in HIV dementia. AIDS Res Hum Retrovir. 2009;25:1071–81.
Salahuddin MF, Qrareya AN, Mahdi F, Jackson D, Foster M, Vujanovic T, et al. Combined HIV-1 Tat and oxycodone activate the hypothalamic-pituitary-adrenal and -gonadal axes and promote psychomotor, affective, and cognitive dysfunction in female mice. Horm Behav. 2020;119:104649.
Paris JJ, Fenwick J, McLaughlin JP. Progesterone protects normative anxiety-like responding among ovariectomized female mice that conditionally express the HIV-1 regulatory protein, Tat, in the CNS. Horm Behav. 2014;65:445–53.
Paris JJ, Zou S, Hahn YK, Knapp PE, Hauser KF. 5α-reduced progestogens ameliorate mood-related behavioral pathology, neurotoxicity, and microgliosis associated with exposure to HIV-1 Tat. Brain Behav Immun. 2016;55:202–14.
Bruce-Keller AJ, Turchan-Cholewo J, Eric J. Smart TG, Chauhan A, Reid R, Xu R, et al. Morphine causes rapid increases in glial activation and neuronal injury in the striatum of inducible HIV-1 Tat transgenic mice. Glia. 2008;1414–1427, 56.
Lucchetti J, Fracasso C, Balducci C, Passoni A, Forloni G, Salmona M, et al. Plasma and brain concentrations of doxycycline after single and repeated doses in wild-type and APP23 mice. J Pharmacol Exp Ther. 2019;368:32–40.
Fitting S, Scoggins KL, Xu R, Dever SM, Knapp PE, Dewey WL, et al. Morphine efficacy is altered in conditional HIV-1 Tat transgenic mice. Eur J Pharmacol. 2012;689:96–103.
Paris JJ, Singh HD, Ganno ML, Jackson P, McLaughlin JP. Anxiety-like behavior of mice produced by conditional central expression of the HIV-1 regulatory protein. Tat Psychopharmacol. 2014;231:2349–60.
Hall C, Ballachey EL. A study of the rat’s behavior in a field. A contribution to method in comparative psychology. Univ Calif Publ Psychol. Berkeley: University of California Press; 1932. pp.1–12.
File SE, Lippa AS, Beer B, Lippa MT. Animal tests of anxiety. Curr Protoc Pharmacol. 2005;5:5.38.
Burešová O, Bureš J, Oitzl MS, Zahálka A. Radial maze in the water tank: an aversively motivated spatial working memory task. Physiol Behav. 1985;34:1003–5.
Sparkman NL, Buchanan JB, Dos Santos NL, Johnson RW, Burton MD. Aging sensitizes male mice to cognitive dysfunction induced by central HIV-1 gp120. Exp Gerontol. 2019;126:110694.
Crabbe JC, Cotnam CJ, Cameron AJ, Schlumbohm JP, Rhodes JS, Metten P, et al. Strain differences in three measures of ethanol intoxication in mice: the screen, dowel and grip strength tests. Genes Brain Behav. 2003;2:201–13.
Hahn YK, Podhaizer EM, Farris SP, Miles MF, Hauser KF, Knapp PE. Effects of chronic HIV-1 Tat exposure in the CNS: heightened vulnerability of males versus females to changes in cell numbers, synaptic integrity, and behavior. Brain Struct Funct. 2015;220:605–23.
Kim S, Chung J. Sympathectomy alleviates mechanical allodynia in an experimental animal model for neuropathy in the rat. Neurosci Lett. 1991;134:131–4.
Wodarski R, Bagdas D, Paris JJ, Pheby T, Toma W, Xu R, et al. Reduced intraepidermal nerve fibre density, glial activation, and sensory changes in HIV type-1 Tat-expressing female mice: involvement of Tat during early stages of HIV-associated painful sensory neuropathy. Pain Rep. 2018;3:e654.
Deuis JR, Vetter I. The thermal probe test: a novel behavioral assay to quantify thermal paw withdrawal thresholds in mice. Temp. 2016;3:199–207.
Paris JJ, Singh HD, Carey AN, McLaughlin JP. Exposure to HIV-1 Tat in brain impairs sensorimotor gating and activates microglia in limbic and extralimbic brain regions of male mice. Behav Brain Res. 2015;291:209–18.
Bagdas D, Paris JJ, Carper M, Wodarksi R, Rice ASC, Knapp PE, et al. Conditional expression of HIV-1 tat in the mouse alters the onset and progression of tonic, inflammatory and neuropathic hypersensitivity in a sex-dependent manner. Eur J Pain. 2020; In Press;24:1609–23. https://doi.org/10.1002/ejp.1618.
Ciesla JA, Roberts JE. Meta-analysis of the relationship between HIV infection and risk for depressive disorders. Am J Psychiatry. 2001;158:725–30.
Morrison MF, Petitto JM, Ten Have T, Gettes DR, Chiappini MS, Weber AL, et al. Depressive and anxiety disorders in women with HIV infection. Am J Psychiatry. 2002;159:789–96.
Nakasujja N, L Skolasky R, Musisi S, Allebeck P, Robertson K, Ronald A, et al. Depression symptoms and cognitive function among individuals with advanced HIV infection initiating HAART in Uganda BMC Psychiatry. 2010;10:44.
Gongvatana A, Schweinsburg BC, Taylor MJ, Theilmann RJ, Letendre S, Alhassoon OM, et al. White matter tract injury and cognitive impairment in human immunodeficiency virus-infected individuals. J Neuro-OncolJ Neurovirol. 2009;15:187–95.
Bendlin BB, Fitzgerald ME, Ries ML, Xu G, Erik K, Thiel BW, et al. White matter in aging and cognition: a cross-sectional study of microstructure in adults aged eighteen to eighty-three. Dev Neuropsychol. 2010;35:257–77.
Harezlak J, Buchthal S, Taylor MD, Schifitto G, Zhong J, Daar ES, et al. Persistence of HIV− associated cognitive impairment, inflammation and neuronal injury in era of highly active antiretroviral treatment. AIDS. 2011;25:625–33.
Ances BM, Ortega M, Vaida F, Heaps J, Paul R. Independent effects of HIV, aging, and HAART on brain volumetric measures. J Acquir Immune Defic Syndr. 2012;59:469–77.
du Plessis S, Vink M, Joska JA, Koutsilieri E, Bagadia A, Stein DJ, et al. Prefrontal cortical thinning in HIV infection is associated with impaired striatal functioning. J Neural Transm. 2016;123:643–51.
Alakkas A, Ellis RJ, Watson CWM, Umlauf A, Heaton RK, Letendre S, et al. White matter damage, neuroinflammation, and neuronal integrity in HAND. J Neuro-Oncol. 2019;25:32–41.
Thompson PM, Dutton RA, Hayashi KM, Toga AW, Lopez OL, Aizenstein HJ, et al. Thinning of the cerebral cortex visualized in HIV/AIDS reflects CD4 + T lymphocyte decline. Proc Natl Acad Sci U S A. 2005;102:15647–52.
O’Connor EE, Zeffiro T, Lopez OL, Becker JT, Zeffiro T. HIV infection and age effects on striatal structure are additive. J Neuro-Oncol. 2019;25:480–95.
Guha A, Brier MR, Ortega M, Westerhaus E, Nelson B, Ances BM. Topographies of cortical and subcortical volume loss in HIV and aging in the cART era. J Acquir Immune Defic Syndr. 2016;73:374–83.
Hinkin .H., Castellon SA, Atkinson JH, Goodkinc K. Neuropsychiatric aspects of HIV infection among older adults. J Clin Epidemiol. 2001;54:44–52.
Grov C, Golub SA, Parsons JT, Brennan M, Karpiak SE. Loneliness and HIV-related stigma explain depression among older HIV-positive adults. AIDS Care. 2010;22:630–9.
Saylor D, Sacktor N. Cognitive impairment among older individuals with HIV infection. Curr Geri Rep. 2016;5:63–70.
Sacktor N. Changing clinical phenotypes of HIV-associated neurocognitive disorders. J Neuro-Oncol. 2018;24:141–5.
Mackiewicz MM, Overk C, Achim CL, Masliah E. Pathogenesis of age-related HIV neurodegeneration. J Neuro-Oncol. 2019;25:622–33.
McLaughlin JP, Paris JJ, Mintzopoulos D, Hymel KA, Kim JK, Cirino TJ, et al. Conditional human immunodeficiency virus transactivator of transcription protein expression induces depression-like effects and oxidative stress. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2:599–609.
Lawson MA, Kelley KW, Dantzer R. Intracerebroventricular administration of HIV-1 Tat induces brain cytokine and indoleamine 2,3-dioxygenase expression: a possible mechanism for AIDS comorbid depression. Brain Behav Immun. 2011;25:1569–75.
Carey AN, Sypeka EI, Singhc HD, Kaufmanb MJ, McLaughlin JP. Expression of HIV-Tat protein is associated with learning and memory deficits in the mouse. Behav Brain Res. 2012;229:48–56.
Carey AN, Liu X, Mintzopoulos D, Paris JJ, Muschamp JW, McLaughlin JP, et al. Conditional Tat protein expression in the GT-tg bigenic mouse brain induces gray matter density reductions. Prog Neuro-Psychopharmacol Biol Psychiatry. 2013;43:49–54.
Carey AN, Xiaoxu Liu DM, Paris JJ, McLaughlin JP, Kaufman MJ. Conditional Tat protein brain expression in the GT-tg bigenic mouse induces cerebral fractional anisotropy abnormalities. Curr HIV Res. 2015;13:3–9.
Marks WD, Paris JJ, Schier CJ, Denton MD, Fitting S, McQuiston AR, et al. HIV-1 Tat causes cognitive deficits and selective loss of parvalbumin, somatostatin, and neuronal nitric oxide synthase expressing hippocampal CA1 interneuron subpopulations. J Neuro-Oncol. 2016;22:747–62.
Cotter AG, Powderly WG. Endocrine complications of human immunodeficiency virus infection: hypogonadism, bone disease and tenofovir-related toxicity. Best Pract Res Clin Endocrinol Metab. 2011;25:501–15.
Tripathy SK, Agrawala RK, Baliarsinha AK. Endocrine alterations in HIV-infected patients. Indian J Endocrinol Metab. 2015;19:143–147.
de Pommerol M, Hessamfar M, Lawson-Ayayi S, Neau D, Geffard S, Farbos S, et al. Menopause and HIV infection: age at onset and associated factors, ANRS CO3 Aquitaine cohort. Int J STD AIDS. 2011;22:67–72.
Looby SE, Shifren J, Corless I, Rope A, Pedersen MC, Joffe H, et al. Increased hot flash severity and related interference in perimenopausal HIV-infected women. Menopause. 2014;21:403–9.
Andany N, Aden v LKM, Loutfy M. Perspectives on menopause and women with HIV. Int J Womens Heal 2016;8:1–22.
Dobs AS. Role of testosterone in maintaining lean body mass and bone density in HIV-infected patients. Int J Impot Res. 2003;15:S21–5.
Laan ETM, Prins JM, van Lunsen RHW, Nieuwkerk PT, Nievaard-Boon MAF. Testosterone insufficiency in human immunodeficiency viruse-infected women: a cross-sectional study. Sex Med. 2018;7:72–9.
Utian WH, Archer DF, Bachmann GA, Gallagher JC, Grodstein F, Heiman SJR, et al. Estrogen and progestogen use in postmenopausal women: July 2008 position statement of the North American Menopause Society. Menopause. 2008;15:584–602.
Rasgon NL, Geist CL, Kenna HA, Wroolie TE, Williams KE, Silverman DHS. Prospective randomized trial to assess effects of continuing hormone therapy on cerebral function in postmenopausal women at risk for dementia. PLoS One. 2014;9:e89095.
Brinton RD, Yao J, Yin F, Mack WJ, Cadenas E. Perimenopause as a neurological transition state. Nat Rev Endocrinol. 2015;11:393–405.
Mukerji SS, Misra V, Lorenz DR, Chettimada S, Keller K, Letendre S, et al. Low neuroactive steroids identifies a biological subtype of depression in adults with human immunodeficiency virus on suppressive antiretroviral therapy. J Infect Dis. 2020; In Press. DOI: jiaa104.
Paris JJ, Liere P, Kim S, Mahdi F, Buchanana ME, Nass SR, et al. Pregnane steroidogenesis is altered by HIV-1 Tat and morphine: physiological allopregnanolone is protective against neurotoxic and psychomotor effects. Neurobiol Stress. 2020;12:2352–895.
Koebele SV, Bimonte-Nelson HA. Modeling menopause: the utility of rodents in translational behavioral endocrinology research. Maturitas. 2016;87:5–17.
Wang JM, Singh C, Liu L, Irwin RW, Chen S, Chung EJ, et al. Allopregnanolone reverses neurogenic and cognitive deficits in mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2010;107:6498–503.
Schumacher M, Mattern C, Ghoumari A, Oudinet JP, Liere P, Labombarda F, et al. Revisiting the roles of progesterone and allopregnanolone in the nervous system: resurgence of the progesterone receptors. Prog Neurobiol. 2014;113:6–39.
Reddy DS, Estes WA. Clinical potential of neurosteroids for CNS disorders. Trends Pharmacol Sci. 2016;37:543–61.
Yilmaz C, Karali K, Fodelianaki G, Gravanis A, Chavakis T, Charalampopoulos I, et al. Neurosteroids as regulators of neuroinflammation. Front Neuroendocrinol. 2019;55:100788.
Meffre D, Pianos A, Liere P, Eychenne B, Cambourg A, Schumacher M, et al. Steroid profiling in brain and plasma of male and pseudopregnant female rats after traumatic brain injury: analysis by gas chromatography/mass spectrometry. Endocrinology. 2007;148:2505–17.
Micevych P, Sinchak K. The neurosteroid progesterone underlies estrogen positive feedback of the LH surge. Front Endocrinol (Lausanne). 2011;2:90.
Wilson ME, Allred KF, Bisotti AJ, Bruce-Keller A, Chuahan A, Nath A. Estradiol negatively regulates HIV-LTR promoter activity in glial cells. AIDS Res Hum Retrovir. 2006;22:350–6.
Bertrand SJ, Mactutus CF, Aksenova MV, Espensen-Sturges TD, Booze RM. Synaptodendritic recovery following HIV Tat exposure: neurorestoration by phytoestrogens. J Neurochem. 2014;128:140–51.
Bertrand SJ, Hu C, Aksenova MV, Mactutus CF, Booze RM. HIV-1 Tat and cocaine mediated synaptopathy in cortical and midbrain neurons is prevented by the isoflavone Equol. Front Microbiol. 2015;6:894.
Moran LM, McLaurin KA, Booze RM, Mactutus CF. Neurorestoration of sustained attention in a model of HIV-1 associated neurocognitive disorders. Front Behav Neurosci. 2019;13:169.
McLaurin KA, Moran LM, Booze RM, Mactutus CF. Selective estrogen receptor β agonists: a therapeutic approach for HIV-1 associated neurocognitive disorders. J NeuroImmune Pharmacol. 2020;15:264–79.
Mediouni S, Jablonski J, Paris JJ, Clementz MA, Thenin-Houssier S, McLaughlin JP, et al. Didehydro-Cortistatin A inhibits HIV-1 Tat mediated neuroinflammation and prevents potentiation of cocaine reward in Tat transgenic mice. Curr HIV Res. 2015;13:64–79.
Mousseau G, Clementz MA, Bakeman WN, Nagarsheth N, Cameron M, Shi J, et al. An analog of the natural steroidal alkaloid Cortistatin A potently suppresses Tat dependent HIV transcription. Cell Host Microbe. 2012;12:97–108.
Mousseau G, Kessing CF, Fromentin R, Trautmann L, Chomont N, Valente T. The Tat inhibitor didehydro-cortistatin A prevents HIV-1. MBio. 2015;6:e00465–15.
Ellis RJ, Rosario D, Clifford DB, McArthur JC, Simpson D, Alexander T, et al. Continued high prevalence and adverse clinical impact of human immunodeficiency virus–associated sensory neuropathy in the era of combination antiretroviral therapy. Arch Neurol. 2010;67:552–8.
Saylor D, Nakigozi G, Nakasujja N, Robertson K, Gray RH, Wawer MJ, et al. Peripheral neuropathy in HIV-infected and uninfected patients in Rakai. Uganda Neurology. 2017;89:485–91.
Tagliati M, Grinnell J, Godbold J, Simpson DM. Peripheral nerve function in HIV infection: clinical, electrophysiologic, and laboratory findings. Arch Neurol. 1999;56:84–9.
Ferrell BA, Ferrell BR, Rivera L. Pain in cognitively impaired nursing home patients. J Pain Symptom Manag. 1995;10:591–8.
Morone NE, Greco CM. Mind-body interventions for chronic pain in older adults: a structured review. Pain Med. 2007;8:359–75.
Sofaer-bennett B, Walker J, Moore A, Lamberty J, Thorp T, O’dwyer J. The social consequences for older people of neuropathic pain: a qualitative study. Pain Med. 2007;8:263–70.
Milligan ED, Mehmert KK, Hinde JL, Jr LOH, Martin D, Tracey KJ, et al. Thermal hyperalgesia and mechanical allodynia produced by intrathecal administration of the human immunodeficiency virus-1 (HIV-1) envelope glycoprotein, gp120. Brain Res. 2000;861:105–16.
Chi X, Amet T, Byrd D, Chang K-H, Shah K, Hu N, et al. Direct effects of HIV-1 Tat on excitability and survival of primary dorsal root ganglion neurons: possible contribution to HIV-1-associated pain. PLoS One. 2011;6:e24412.
Yuan S, Shi Y, Chen J, Zhou X, Li G, Gelman BB, et al. Gp120 in the pathogenesis of human HIV-associated pain. Ann Neurol. 2014;75:837–50.
Guindon J, Blanton H, Brauman S, Donckels K, Narasimhan M, Benamar K. Sex differences in a rodent model of HIV-1-associated neuropathic pain. Int J Mol Sci. 2019;20:1196. Published 2019 Mar 9.
Wallace VCJ, Blackbeard J, Pheby T, Segerdahl AR, Davies M, Hasnie F, et al. Pharmacological, behavioural and mechanistic analysis of HIV-1 gp120 induced painful neuropathy. Pain. 2007;133:47–63.
Wu B, Peng L, Xie J, Zou L, Zhu Q, Jiang H, et al. The P2X7 receptor in dorsal root ganglia is involved in HIV gp120-associated neuropathic pain. Brain Res Bull. 2017;135:25–32.
Takahashi K, Yi H, Liu CH, Liu S, Kashiwagi Y, Patin DJHS. Spinal bromodomain-containing protein 4 contributes to neuropathic pain induced by HIV glycoprotein 120 with morphine in rats. Neuroreport. 2018;29:441–6.
Yi Z, Ouyang S, Zhou C, Xie L, Fang Z, Yuan H, et al. Andrographolide inhibits mechanical and thermal hyperalgesia in a rat model of HIV-induced neuropathic pain. Front Pharmacol. 2018;9:593.
Johnstone TBC, Xie JY, Qu C, Wasiak DJ, JHogenkamp D, Porreca F, et al. Positive allosteric modulators of non-benzodiazepine γ- aminobutyric acidA receptor subtypes for the treatment of chronic pain. Pain. 2019;160:198–209.
Martina C, Solders G, Sönnerborg A, Hansson P. Painful and non-painful neuropathy in HIV-infected patients: an analysis of somatosensory nerve function. Eur J Pain. 2003;7:23–31.
Bhasin S, Singh AB, Javanbakht M. Neuroendocrine abnormalities associated with HIV infection. Endocrinol Metab Clin N Am. 2001;30:749–64.
Grinspoon S. Androgen deficiency and HIV infection. Clin Infect Dis. 2005;41:1804–5.
Ashby J, Goldmeier D, Sadeghi-Nejad H. Hypogonadism in human immunodeficiency virus-positive men. Korean J Urol. 2014;55:9–16.
Rabkin JG, McElhiney MC, Rabkin R, McGrath PJ, Ferrando SJ. Placebo-controlled trial of dehydroepiandrosterone (DHEA) for treatment of nonmajor depression in patients with HIV/AIDS. Am J Psychiatry. 2006;163:59–66.
Miller K, Corcoran C, Armstrong C, Caramelli K, Anderson E, Cotton D, et al. Transdermal testosterone administration in women with acquired immunodeficiency syndrome wasting: a pilot study. J Clin Endocrinol Metab. 1998;83:2717–25.
Acknowledgments
This work was supported by funds from the University of Mississippi and the National Institutes of Health: R00 DA039791 (JJP), R01 DA039044 (MJK), and an administrative supplement from award P30 GM122733 (pilot project to JJP).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1718 kb)
About this article
Cite this article
Qrareya, A.N., Mahdi, F., Kaufman, M.J. et al. HIV-1 Tat promotes age-related cognitive, anxiety-like, and antinociceptive impairments in female mice that are moderated by aging and endocrine status. GeroScience 43, 309–327 (2021). https://doi.org/10.1007/s11357-020-00268-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-020-00268-z