Abstract
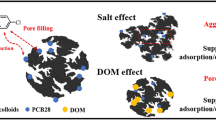
Biochar (BC) colloids attract increasing interest due to their unique environmental behavior and potential risks. However, the interaction between BC colloids and organic contaminants that may affect their fates in the environment has not been substantially studied. Herein, adsorption and desorption of phenanthrene (PHN), atrazine (ATZ), and oxytetracycline (OTC) by a series of BC colloids derived from bulk rice straw BC samples with 6 pyrolysis temperatures (200–700 °C), and 3 particle sizes (250 nm, 500 nm, and 1 μm) were investigated. Regardless of pyrolysis temperature, BC colloids from a given sized bulk BC had a comparable size, being 30 ± 6, 70 ± 18, and 140 ± 15 nm corresponding to the three sized bulk BCs, respectively. The adsorption kinetics curves were well explained by the pseudo-second-order model, and pore diffusion was the primary rate-determining step. Both Freundlich and Langmuir models well fitted the adsorption isotherms. With increasing pyrolysis temperature or decreasing particle size of bulk BC, the specific surface area and pore volumes of the derived BC colloids increased, the kinetics model fitted adsorption rates (k2) of the three organics by the BC colloids all largely decreased, and the Langmuir model fitted adsorption capacities (Qmax) increased. The highest Qmax was obtained by BC colloids from the smallest (250 nm) bulk BC with the highest pyrolysis temperature (700 °C), being 212 μmol g−1 for PHN, 815 μmol g−1 for ATZ, and 72.4 μmol g−1 for OTC. The adsorption was reversible for PHN and ATZ, while significant desorption hysteresis was observed for OTC on BC colloids with middle pyrolysis temperatures (300–500 °C). The underlying mechanisms including hydrophobic interaction, π–π electron donor-acceptor interaction, molecular size effect, and irreversible reactions were discussed to explain the difference in the adsorption and desorption behaviors. The findings increased our understanding of the environmental fate and risk of BC.






Similar content being viewed by others
References
ASTM (1989) Standard methods for chemical analysis of wood charcoal. American Society for Testing and Materials, Philadelphia
Ahmad M, Rajapaksha AU, Lim JE, Zhang M, Bolan N, Mohan D, Vithanage M, Lee SS, Ok YS (2014) Biochar as a sorbent for contaminant management in soil and water: a review. Chemosphere 99:19–33
Agrafioti E, Bouras G, Kalderis D, Diamadopoulos E (2013) Biochar production by sewage sludge pyrolysis. J Anal Appl Pyrolysis 101:72–78
Apul OG, Karanfil T (2015) Adsorption of synthetic organic contaminants by carbon nanotubes: a critical review. Water Res 68:34–55
Barriuso E (1994) Atrazine desorption from smectites. Soil Sci Soc Am J 58(6):1632–1638
Chen BL, Zhou DD, Zhu LZ (2008) Transitional adsorption and partition of nonpolar and polar aromatic contaminants by biochars of pine needles with different pyrolytic temperature. Environ Sci Technol 42:5137–5143
Ferrari AC (2007) Raman spectroscopy of graphene and graphite: disorder, electron–phonon fixed-bed column study. Chem Eng J 228:496–505
Fu H, Wei C, Qu X, Li H, Zhu D (2018) Strong binding of apolar hydrophobic organic contaminants by dissolved black carbon released from biochar: a mechanism of pseudo micelle partition and environmental implications. Environ Pollut 232:402–410
Hameed R, Cheng LL, Yang K, Fang J, Lin DH (2019) Endogenous release of metals with dissolved organic carbon from biochar: effects of pyrolysis temperature, particle size, and solution chemistry. Environ Pollut 255(2):1–10
Huang W, Yu H, Weber WJ (1998) Hysteresis in the sorption and desorption of hydrophobic organic contaminants by soils and sediments. 1. A comparative analysis of experimental protocols. J Contam Hydrol 31:129–148
Ji L, Chen W, Duan L, Zhu D (2009) Mechanisms for strong adsorption of tetracycline to carbon nanotubes: a comparative study using activated carbon and graphite as adsorbents. Environ Sci Technol 43(7):2322–2327
Jia M, Wang F, Bian Y, Jin X, Song Y, Kengara FO, Xu R, Jiang X (2013) Effects of pH and metal ions on oxytetracycline sorption to maize-straw-derived biochar. Bioresour Technol 136:87–93
Jones KD, Tiller CL (1999) Effect of solution chemistry on the extent of binding of phenanthrene by a soil humic acid: a comparison of dissolved and clay bound humic. Environ Sci Technol 33(4):580–587
Keiluweit M, Nico PS, Johnson MG, Kleber M (2010) Dynamic molecular structure of plant biomass-derived black carbon(biochar). Environ Sci Technol 44:1247–1253
Lazar P, Karlický F, Jurečka P, Kocman M, Otyepková E, Šafářová K, Otyepka M (2013) Adsorption of small organic molecules on graphene. J Am Chem Soc 135:6372–6377
Lehmann J, Joseph S (2009) Biochar for environmental management: science and technology. Earthscan Publications Ltd., London
Li Y, Li M, Li Z, Yang L, Liu X (2019) Effects of particle size and solution chemistry on triclosan sorption on polystyrene microplastic. Chemosphere 231:308–314
Li S, Lü J, Zhang T, Cao Y, Li Y (2017) Relationship between biochars' porosity and adsorption of three neutral herbicides from water. Water Sci Technol 75:482–489
Lin DH, Pan B, Zhu LZ, Xing BS (2007) Characterization and phenanthrene sorption of tea leaf powders. J Agric Food Chem 55:5718–5724
Liu G, Zheng H, Jiang Z, Zhao J, Wang Z, Pan B, Xing B (2018) Formation and physicochemical characteristics of nano biochar: insight into chemical and colloidal stability. Environ Sci Technol 52(18):10369–10379
Liu Z, Zhang FS, Wu J (2010) Characterization and application of chars produced from pyrolysis and hydrothermal treatment. Fuel 89:510–551
Major J, Lehmann J, Rondon M, Goodale C (2010) Fate of soil- applied black carbon: downward migration, leaching and soil respiration. Glob Chang Biol 16:1366–1379
Mukherjee S, Weihermüller L, Tappe W, Hofmann D, Köppchen S, Laabs V, Vereecken H, Burauel P (2016) Sorption–desorption behaviour of bentazone, boscalid and pyrimethanil in biochar and digestate based soil mixtures for biopurification systems. Sci Total Environ 559:63–73
Oleszczuk P, Pan B, Xing BS (2009) Adsorption and desorption of oxytetracycline and carbamazepine by multiwalled carbon nanotubes. Environ Sci Technol 43:9167–9173
Qian L, Zhang W, Yan J, Han L, Gao W, Liu R, Chen M (2016) Effective removal of heavy metal by biochar colloids under different pyrolysis temperatures. Bioresour Technol 206:217–224
Qu XL, Fu HY, Mao JD, Ran Y, Zhang DN, Zhu DQ (2016) Chemical and structural properties of dissolved black carbon released from biochars. Carbon 96:759–767
Schlautman MA, MorganJJ (1993) Effects of aqueous chemistry on the binding of polycyclic aromatic hydrocarbons by dissolved humic materials. Environ Sci Technol 27(5):961–969
Spokas KA, Novak JM, Masiello CA, Johnson MG, Colosky EC, Trigo JA, Trigo C (2014) Physical disintegration of biochar: an overlooked process. Environ Sci Technol Lett 1:326–332
Sun W, Yin K, Yu X (2013) Effect of natural aquatic colloids on Cu (II) and Pb (II) adsorption by Al2O3 nanoparticles. Chem Eng J 225:464–473
Wang XL, Xing B (2007) Sorption of organic contaminants by biopolymer-derived chars. Environ Sci Technol 41:8342–8348
Wang Y, Li Y, Kim H, Walker SL, Abriola LM, Pennell KD (2010) Transport and retention of fullerene nanoparticles in natural soils. J Environ Qual 39(6):1925–1933
Wang D, Zhang W, Hao X, Zhou D (2013) Transport of biochar particles in saturated granular media: effects of pyrolysis temperature and particle size. Environ Sci Technol 47:821–828
Wang S, Gao B, Zimmerman AR, Li Y, Ma L, Harris WG, Migliaccio KW (2015) Physicochemical and sorptive properties of biochars derived from woody and herbaceous biomass. Chemosphere 134:257–262
Wang P, Tang L, Wei X, Zeng G, Zhou Y, Deng Y, Wang J, Xie Z, Fang W (2017) Synthesis and application of iron and zinc-doped biochar for removal of p-nitrophenol in wastewater and assessment of the influence of co-existed Pb(II). Appl Surf Sci 392:391–401
Wu L, Lin L, Gao B, Rafael MC, Zhang M, Chen H, Zhou Z, Wang H (2013) Aggregation kinetics of graphene oxides in aqueous solutions: experiments, mechanisms, and modelling. Langmuir 29:15174–15181
Xie M, Chen W, Xu Z, Zheng S, Zhu D (2014) Adsorption of sulfonamides to demineralized pine wood biochars prepared under different thermochemical conditions. Environ Pollut 186:187–194
Yah LG, Qin LL, Yu HQ, Li S, Shan RR, Du B (2016) Adsorption of acid dyes from aqueous solution by CTMAB modified bentonite: kinetic and isotherm modeling. J Mol Liq 211:1074–1081
Yang K, Yang JJ, Jiang Y, Wu WH, Lin DH (2016) Correlations and adsorption mechanisms of aromatic compounds on a high heat temperature treated bamboo biochar. Environ Pollut 210:57–64
Yang K, Jiang Y, Yang J, Lin DH (2018) Correlations and adsorption mechanisms of aromatic compounds on biochars produced from various biomass at 700 °C. Environ Pollut 233:64–70
Yang L, Zhao H, Liu N, Wang W (2019) A target analyte induced fluorescence band shift of piperazine modified carbon quantum dots: a specific visual detection method for oxytetracycline. Chem Commun 82(55):12364–12367
Zhang P, Sun H, Yu L, Sun T (2013) Adsorption and catalytic hydrolysis of carbaryl and atrazine on pig manure-derived biochars: impact of structural properties of biochars. J Hazard Mater 244–245:217–224
Zhang P, Sun H, Yu L, Sun T (2012) Adsorption and catalytic hydrolysis of carbaryl and atrazine on pig manure-derived biochars: impact of structural properties of biochars. J Hazard Mater 245:217–224
Zhang W, Niu J, Morales VL, Chen X, Hay AG, Lehmann J, Steenhuis TS (2010) Transport and retention of biochar particles in porous media: effect of pH, ionic strength, and particle size. Ecohydrology 3(4):497–508
Zhao X, Ouyang W, Hao F, Lin C, Wang F, Han S, Geng X (2013a) Properties comparison of biochars from corn straw with different pretreatment and sorption behaviour of atrazine. Bioresour Technol 147:338–344
Zhao X, Ouyang W, Hao F, Lin C, Wang F, Han S, Geng X (2013b) Properties comparison of biochars from corn straw with different pretreatment and sorption behaviour of atrazine. Bioresour Technol 147:338–344
Zhu DQ, Kwon S, Pignatelli JJ (2005) Adsorption of single-ring organic compounds to wood charcoals prepared under different thermochemical conditions. Environ Sci Technol 39:3990–3939
Zhu D, Pignatello JJ (2005) Characterization of aromatic compound sorptive interactions with black carbon (charcoal) assisted by graphite as a model. Environ Sci Technol 39:2033–2041
Zhu X, Li C, Li J, Xie B, Lü J, Li Y (2018) Thermal treatment of biochar in the air/nitrogen atmosphere for developed mesoporosity and enhanced adsorption to tetracycline. Bioresour Technol 263:475–482
Funding
This work was supported by the National Key Research and Development Program of China (2017YFA0207003) and the National Natural Science Foundation of China (21621005 and 21525728).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Zhihong Xu
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 4620 kb)
Rights and permissions
About this article
Cite this article
Hameed, R., Lei, C. & Lin, D. Adsorption of organic contaminants on biochar colloids: effects of pyrolysis temperature and particle size. Environ Sci Pollut Res 27, 18412–18422 (2020). https://doi.org/10.1007/s11356-020-08291-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-08291-5