Abstract
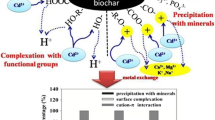
We quantified and investigated mechanisms for Cd2+ adsorption on biochars produced from plant residual and animal waste at various temperatures. Ten biochars were produced by pyrolysis of rice straw (RB) and swine manure (SB) at 300–700 °C and characterized. The Cd2+ adsorption isotherms, adsorption kinetics, and desorption characteristics were studied via a series of batch experiments, and Cd2+-loaded biochars were analyzed by SEM–EDS and XRD. The total Cd2+ adsorption capacity (Qc) increased with pyrolysis temperature for both biochars, however, rice straw-derived biochars had greater Qc than swine manure-derived biochars; hence, the biochar derived from rice straw at 700 °C (RB700) had the largest Qc, 64.4 mg g−1, of all studied biochars. Cadmium adsorption mechanisms in this study involved precipitation with minerals (Qcp), cation exchange (Qci), complexation with surface functional groups (Qco), and Cd-π interactions (Qcπ). Both the pyrolysis temperature and feedstock affected the quantitative contributions of the various adsorption mechanisms. The relative percent contributions to Qc for Cd2+ adsorption by RB and SB were 32.9–72.9% and 35.0–72.5% for Qcp, 21.7–50.9% and 20.4–43.3% for Qci, 2.2–14.8% and 1.4–18.8% for Qco, and 1.4–3.1% and 3.0–5.8% for Qcπ, respectively. For biochars produced at higher pyrolysis temperatures, the contributions of Qcp and Qcπ to adsorption increased, while the contributions of Qci and Qco decreased. Generally, Qcp dominated Cd2+ adsorption by high-temperature biochars (700 °C) (accounting for approximately 73% of Qc), and Qci was the most prominent mechanism for low-temperature biochars (400 °C) (accounting for 43.3–50.9% of Qc). Results suggested that biochar derived from rice straw is a promising adsorbent for the Cd2+ removal from wastewater and that the low-temperature biochars may outperform the high-temperature biochars for Cd2+ immobilization in acidic water or soils.






Similar content being viewed by others
References
Ahmad M, Rajapaksha AU, Lim JE, Zhang M, Bolan N, Mohan D, Vithanage M, Lee SS, Ok YS (2014) Biochar as a sorbent for contaminant management in soil and water: a review. Chemosphere 99:19–33
Ali I, Gupta VK (2007) Advances in water treatment by adsorption technology. Nat Protoc 1:2661–2667
Banik C, Lawrinenko M, Bakshi S, Laird DA (2018) Impact of pyrolysis temperature and feedstock on surface charge and functional group chemistry of biochars. J Environ Qual 47:452–461
Bashir S, Zhu J, Fu Q, Hu H (2018) Comparing the adsorption mechanism of Cd by rice straw pristine and KOH-modified biochar. Environ Sci Pollut Res Int 25:11875–11883
Beesley L, Morenojimenez E, Gomezeyles JL, Harris E, Robinson B, Sizmur T (2011) A review of biochars’ potential role in the remediation, revegetation and restoration of contaminated soils. Environ Pollut 159:3269–3282
Cantrell KB, Hunt PG, Uchimiya M, Novak JM, Ro KS (2012) Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour Technol 107:419–428
Cao X, Harris W (2010) Properties of dairy-manure-derived biochar pertinent to its potential use in remediation. Bioresour Technol 101:5222–5228
Cao X, Ma L, Gao B, Harris W (2009) Dairy-manure derived biochar effectively sorbs lead and atrazine. Environ Sci Technol 43:3285–3291
Chen B, Zhou D, Zhu L (2008) Transitional adsorption and partition of nonpolar and polar aromatic contaminants by biochars of pine needles with different pyrolytic temperatures. Environ Sci Technol 42:5137–5143
Chen T, Zhang Y, Wang H, Lu W, Zhou Z, Ren L (2014) Influence of pyrolysis temperature on characteristics and heavy metal adsorptive performance of biochar derived from municipal sewage sludge. Bioresour Technol 164:47–54
Chen Z, Liu T, Tang J, Zheng Z, Wang H, Shao Q, Chen G, Li Z, Chen Y, Zhu J, Feng T (2018) Characteristics and mechanisms of cadmium adsorption from aqueous solution using lotus seedpod-derived biochar at two pyrolytic temperatures. Environ Sci Pollut Res 25:11854–11866
Cui X, Fang S, Yao Y, Li T, Ni Q, Yang X, He Z (2016) Potential mechanisms of cadmium removal from aqueous solution by Canna indica derived biochar. Sci Total Environ 562:517–525
Deng J, Liu Y, Liu S, Zeng G, Tan X, Huang B, Tang X, Wang S, Hua Q, Yan Z (2017) Competitive adsorption of Pb (II), Cd (II) and Cu (II) onto chitosan-pyromellitic dianhydride modified biochar. J Colloid Interface Sci 506:355–364
Fidel RB, Laird DA, Thompson ML, Lawrinenko M (2017) Characterization and quantification of biochar alkalinity. Chemosphere 167:367–373
Giles CH, Smith D, Huitson A (1974) A general treatment and classification of the solute adsorption isotherm. I. Theoretical. J Colloid Interface Sci 47(3):755–765
Han L, Sun H, Ro KS, Sun K, Libra JA, Xing B (2017) Removal of antimony (III) and cadmium (II) from aqueous solution using animal manure-derived hydrochars and pyrochars. Bioresour Technol 234:77–85
Harvey OR, Herbert BE, Rhue RD, Kuo L (2011) Metal interactions at the biochar-water interface: energetics and structure-sorption relationships elucidated by flow adsorption microcalorimetry. Environ Sci Technol 45:5550–5556
Higashikawa FS, Conz RF, Colzato M, Cerri CEP, Alleoni LRF (2016) Effects of feedstock type and slow pyrolysis temperature in the production of biochars on the removal of cadmium and nickel from water. J Clean Prod 137:965–972
Ho YS, Mckay G (1998) A comparison of chemisorption kinetic models applied to pollutant removal on various sorbent. Process Saf Environ Prot 76:332–340
Jazini R, Soleimani M, Mirghaffari N (2018) Characterization of barley straw biochar produced in various temperatures and its effect on lead and cadmium removal from aqueous solutions. Water Environ J 32:125–133
Jiang TY, Xu RK, Gu TX, Jiang J (2014) Effect of crop-straw derived biochars on Pb (II) adsorption in two variable charge soils. J Integr Agric 13:507–516
Jin J, Li S, Peng X, Liu W, Zhang C, Yang Y, Han L, Du Z, Sun K, Wang X (2018) HNO3 modified biochars for uranium (VI) removal from aqueous solution. Bioresour Technol 256:247–253
Keiluweit PSN, Mark GJ, Kleber M (2010) Dynamic molecular structure of plant biomass-derived black carbon (biochar). J Environ Chem Eng 44:1247–1253
Kim WK, Shim T, Kim YS, Hyun S, Ryu C, Park YK, Jung J (2013) Characterization of cadmium removal from aqueous solution by biochar produced from a giant Miscanthus at different pyrolytic temperatures. Bioresour Technol 138:266–270
Kołodyńska D, Wnętrzak R, Leahy JJ, Hayes MHB, Kwapiński W, Hubicki Z (2012) Kinetic and adsorptive characterization of biochar in metal ions removal. Chem Eng J 197:295–305
Lawrinenko M, Laird DA (2015) Anion exchange capacity of biochar. Green Chem 17:4628–4636
Lee SJ, Park JH, Ahn YT, Chung JW (2015) Comparison of heavy metal adsorption by peat moss and peat moss-derived biochar produced under different carbonization conditions. Water Air Soil Pollut 226:1–11
Li R, Liang W, Huang H, Jiang S, Guo D, Li M, Zhang Z, Ali A, Wang JJ (2018) Removal of cadmium (II) cations from an aqueous solution with aminothiourea chitosan strengthened magnetic biochar. J Appl Polym Sci 135:1–11
Liu L, Fan S (2018) Removal of cadmium in aqueous solution using wheat straw biochar: effect of minerals and mechanism. Environ Sci Pollut Res Int 25:8688–8700
Lu H, Zhang W, Yang Y, Huang X, Wang S, Qiu R (2012) Relative distribution of Pb2+ sorption mechanisms by sludge-derived biochar. Water Res 46:854–862
Ma Z, Yang Y, Ma Q, Zhou H, Luo X, Liu X, Wang S (2017) Evolution of the chemical composition, functional group, pore structure and crystallographic structure of bio-char from palm kernel shell pyrolysis under different temperatures. J Anal Appl Pyrolysis 127:350–359
Mahdi Z, Yu QJ, El Hanandeh A (2018) Investigation of the kinetics and mechanisms of nickel and copper ions adsorption from aqueous solutions by date seed derived biochar. J Environ Chem Eng 6:1171–1181
Meng J, Feng X, Dai Z, Liu X, Wu J, Xu J (2014) Adsorption characteristics of Cu (II) from aqueous solution onto biochar derived from swine manure. Environ Sci Pollut Res Int 21:7035–7046
Ministry of Environmental Protection, Ministry of Land and Resources (2014) The national survey of soil pollution. Available http://www.zhb.gov.cn/gkml/hbb/qt/201404/t20140417_270670.htm
Mohammadi N, Khani H, Gupta VK, Amereh E, Agarwal S (2011) Adsorption process of methyl orange dye onto mesoporous carbon material-kinetic and thermodynamic studies. J Colloid Interface Sci 362:457–462. https://doi.org/10.1016/j.jcis.2011.06.067
Pan J, Jiang J, Xu R (2013) Adsorption of Cr (III) from acidic solutions by crop straw derived biochars. J Environ Sci 25:1957–1965
Park JH, Ok YS, Kim SH, Cho JS, Heo JS, Delaune RD, Seo DC (2016) Competitive adsorption of heavy metals onto sesame straw biochar in aqueous solutions. Chemosphere 142:77–83
Peng H, Gao P, Chu G, Pan B, Peng J, Xing B (2017) Enhanced adsorption of Cu (II) and Cd (II) by phosphoric acid-modified biochars. Environ Pollut 229:846–853
Rafiq MT, Aziz R, Yang X, Xiao W, Rafiq MK, Ali B, Li T (2014) Cadmium phytoavailability to rice (Oryza sativa L.) grown in representative Chinese soils. A model to improve soil environmental quality guidelines for food safety. Ecotoxicol Environ Saf 103:101–107
Saeed A, Iqbal M, Akhtar MW (2005) Removal and recovery of lead (II) from single and multimetal (Cd, Cu, Ni, Zn) solutions by crop milling waste (black gram husk). J Hazard Mater 117:65–73
Shi T, Jia S, Chen Y, Wen Y, Du C, Guo H, Wang Z (2009) Adsorption of Pb (II), Cr (III), Cu (II), Cd (II) and Ni (II) onto a vanadium mine tailing from aqueous solution. J Hazard Mater 169:838–846
Shinogi Y, Kanri Y (2003) Pyrolysis of plant, animal and human waste: physical and chemical characterization of the pyrolytic products. Bioresour Technol 90:241–247
Sposito (1989) The chemistry of soils. Oxford University Press, New York (NY)
Tran HN, You SJ, Chao HP (2016) Effect of pyrolysis temperatures and times on the adsorption of cadmium onto orange peel derived biochar. Waste Manag Res 34:129–138
Tran HN, You SJ, Hosseini-Bandegharaei A, Chao HP (2017) Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: a critical review. Water Res 120:88–116
Uchimiya M, Lima IM, Klasson KT, Chang S, Wartelle LH, Rodgers JE (2010) Immobilization of heavy metal ions (CuII, CdII, NiII, and PbII) by broiler litter-derived biochars in water and soil. J Agric Food Chem 58:5538–5544
Uchimiya M, Wartelle LH, Klasson KT, Fortier CA, Lima IM (2011) Influence of pyrolysis temperature on biochar property and function as a heavy metal sorbent in soil. J Agric Food Chem 59:2501–2510
Wang Z, Liu G, Zheng H, Li F, Ngo HH, Guo W, Liu C, Chen L, Xing B (2015) Investigating the mechanisms of biochar’s removal of lead from solution. Bioresour Technol 177:308–317
Wang Z, Shen F, Shen D, Jiang Y, Xiao R (2017) Immobilization of Cu(2+) and Cd(2+) by earthworm manure derived biochar in acidic circumstance. J Environ Sci (China) 53:293–300
Wang RZ, Huang DL, Liu YG, Zhang C, Lai C, Zeng GM, Cheng M, Gong XM, Wan J, Luo H (2018) Investigating the adsorption behavior and the relative distribution of Cd(2+) sorption mechanisms on biochars by different feedstock. Bioresour Technol 261:265–271
Xu R, Xiao S, Zhao A, Ji G (2005) Effect of Cr (VI) anions on adsorption and desorption behavior of Cu (II) in the colloidal systems of two authentic variable charge soils. J Colloid Interface Sci 284:22–29
Xu X, Cao X, Zhao L (2013a) Comparison of rice husk- and dairy manure-derived biochars for simultaneously removing heavy metals from aqueous solutions: role of mineral components in biochars. Chemosphere 92:955–961
Xu X, Cao X, Zhao L, Wang H, Yu H, Gao B (2013b) Removal of Cu, Zn, and Cd from aqueous solutions by the dairy manure-derived biochar. Environ Sci Pollut Res Int 20:358–368
Xu D, Zhao Y, Sun K, Gao B, Wang Z, Jin J, Zhang Z, Wang S, Yan Y, Liu X, Wu F (2014) Cadmium adsorption on plant- and manure-derived biochar and biochar-amended sandy soils: impact of bulk and surface properties. Chemosphere 111:320–326
Yuan JH, Xu RK, Zhang H (2011) The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour Technol 102:3488–3497
Zhang W, Mao S, Chen H, Huang L, Qiu R (2013) Pb (II) and Cr (VI) sorption by biochars pyrolyzed from the municipal wastewater sludge under different heating conditions. Bioresour Technol 147:545–552
Zhang F, Wang X, Yin D, Peng B, Tan C, Liu Y, Tan X, Wu S (2015) Efficiency and mechanisms of Cd removal from aqueous solution by biochar derived from water hyacinth (Eichornia crassipes). J Environ Manag 153:68–73
Funding
This work was jointly supported by the National Key Technology Research and Development Program of the Ministry of Science and Technology of China (No. 2015BAD05B02) and the National Natural Science Foundation of China (No. 51109166)
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Tito Roberto Cadaval Jr
Electronic supplementary material
ESM 1
(DOC 2266 kb)
Rights and permissions
About this article
Cite this article
Deng, Y., Huang, S., Laird, D.A. et al. Quantitative mechanisms of cadmium adsorption on rice straw- and swine manure-derived biochars. Environ Sci Pollut Res 25, 32418–32432 (2018). https://doi.org/10.1007/s11356-018-2991-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2991-1