Abstract
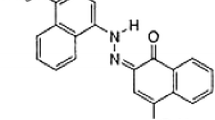
The presence of dyes in water is undesirable due to the toxicological impact of their entrance into the food chain. Owing to the recalcitrant nature of dyes to biological oxidation, a tertiary treatment like adsorption is required. In the present study, unsaturated polyester resin (UPR) has been used as a sorbent in the treatment of dye-contaminated water. Different concentrations of Tropaeoline 000 containing water were treated with UPR. The preliminary investigations were carried out by batch adsorption to examine the effects of pH, adsorbate concentration, adsorbent dosage, contact time, and temperature. A plausible mechanism for the ongoing adsorption process and thermodynamic parameters have also been obtained from Langmuir and Freundlich adsorption isotherm models. Thermodynamic parameter showed that the sorption process of Tropaeoline 000 onto activated carbon (AC) and UPR were feasible, spontaneous, and endothermic under studied conditions. The estimated values for (ΔG) are −10.48 × 103 and −6.098 × 103 kJ mol−1 over AC and UPR at 303 K (30 °C), indicating towards a spontaneous process. The adsorption process followed pseudo-first-order model. The mass transfer property of the sorption process was studied using Lagergren pseudo-first-order kinetic models. The values of % removal and k ad for dye systems were calculated at different temperatures (303–323 K). The mechanism of the adsorption process was determined from the intraparticle diffusion model.







Similar content being viewed by others
References
Ajmal M, Rao RAK, Anwar S, Ahmad J, Ahmad R (2003) Adsorption studies on rice husk: removal and recovery of Cd (II) from wastewater. Biores Technol 86:147–149
APHA (1995) Standard methods for water and wastewater examination. 19th edn. Am Public Health Association, Washington DC
Baccar R, Feki M, Montiel A (2009) Preparation of activated carbon from Tunisian olive waste cake and application for adsorption of heavy metals. J Hazard Mater 162:1522–1529
Chingombe P, Saha B, Wakeman RJ (2006) Sorption of atrazine on conventional and surface modified activated carbons. J Coll Interf Sci 302:408–416
Chu W, Ma CW (2000) Quantitative prediction of direct and indirect dye ozonation kinetics. Water Res 34:3153–3160
Dogan M, Ozdemir Y, Alkan M (2007) Adsorption kinetics and mechanism of cationic methyl violet and methylene blue dyes onto sepiolite. Dyes Pigments 75:701–713
Freundlich HMF (1906) Over the adsorption in solution. J Phy Chem 57:385–470
Goyal RN, Gupta VK, Sangal A, Bachheti N (2005) Voltammetric determination of uric acid at a fullerene-C 60-modified glassy carbon electrode. Electroanalysis 17:2217–2223
Goyal RN, Gupta VK, Oyama M, Bachheti N (2007a) Voltammetric determination of adenosine and guanosine using fullerene-C60-modified glassy carbon electrode. Talanta 71:1110–1117
Goyal RN, Gupta VK, Oyama M, Bachheti N (2007b) Gold nanoparticles modified indium tin oxide electrode for the simultaneous determination of dopamine and serotonin: application in pharmaceutical formulations and biological fluids. Talanta 72:976–983
Goyal RN, Oyama M, Gupta VK, Singh SP, Sharma RA (2008) Sensors for 5-hydroxytryptamine and 5-hydroxyindole acetic acid based on nanomaterial modified electrodes. Sensors and Actuators, B: Chemical 134:816–821
Gupta VK (2006) Removal and recovery of the hazardous azo dye Acid Orange 7 through adsorption over waste materials: bottom ash and de-oiled soya. Ind Eng Chem Res 45:1446–1453
Gupta VK, Mittal A, Jain R, Mathur M, Sikarwar S (2006) Adsorption of Safranin T from wastewater using waste materials-activated carbon and activated rice husk. J Coll Interf Sci 303:80–86
Gupta VK, Al Khayat M, Singh AK, Pal MK (2009a) Nano level detection of Cd(II) using poly(vinyl chloride) based membranes of Schiff bases. Anal Chim Acta 634:36–43
Gupta VK, Mittal A, Kaur D, Malviya A, Mittal J (2009b) Adsorption studies on the removal of colouring agent phenol red from wastewater using waste materials as adsorbents. J Coll Interf Sci 337:345–354
Gupta VK, Mittal A, Malviya A, Mittal J (2009c) Adsorption of carmoisine A from wastewater using waste material—bottom ash and de-oiled soya. J Coll Interf Sci 355:24–33
Gupta VK, Goyal RN, Sharma RA (2009d) Novel PVC membrane based alizarin sensor and its application: determination of vanadium, zirconium and molybdenum. Int J Electrochem Sci 4:156–172
Gupta VK, Ali I (2008) Removal of endosulfan and methoxychlor from water on carbon slurry. Environ Sci Technol 42:766–770
Gupta VK, Jain R, Varshney S (2007a) Removal of Reactofix golden yellow 3 RFN from aqueous solution using wheat husk An agricultural waste. J Hazard Mater 142:443–448
Gupta VK, Ali I, Saini VK (2007b) Adsorption studies on the removal of Vertigo Blue 49 and Orange DNA13 from aqueous solutions using carbon slurry developed from a waste material. 315:87–93
Gupta VK, Jain R, Mittal A, Mathur M, Sikarwar S (2007c) Photochemical degradation of the hazardous dye Safranin-T using TiO2 catalyst. J Coll Interf Sci 309:464–469
Gupta VK, Jain R, Varshney S (2007d) Electrochemical removal of hazardous dye Reactofix Red 3 BFN from industrial effluents. J Coll Interf Sci 312:292–296
Gupta VK, Singh AK, Gupta B (2007e) Schiff bases as cadmium(II) selective ionophores in polymeric membrane electrodes. Anal Chim Acta 583:340–348
Gupta VK, Jain CK, Ali I, Chandra S, Agarwal S (2002a) Removal of lindane and malathion from wastewater using baggase flyash—a sugar industry waste. Water Res 36:2483–2490
Gupta VK, Mittal A, Gajbe V, Krishnan L (2002b) Adsorption kinetics and column operations for the removal and recovery of malachite green from wastewater using bottom ash. Sep Purif Technol 40:87–96
Gupta VK, Mittal A, Gajbe V, Krishnan L (2002c) Removal and recovery of malachite green from wastewater using an agricultural waste material, de-oiled soya. Sep Purif Technol 43:125–133
Gupta VK, Mangla R, Agarwal S (2002d) Pb(II) selective potentiometric sensor based on 4-tert-butylcalix [4] arene in PVC matrix. Electroanalysis 14:1127–1132
Gupta VK, Jain R, Shrivastava M, Nayak A (2010) Equilibrium and thermodynamic studies on the adsorption of the dye Tartrazine onto waste “Coconut Husks” carbon and activated Carbon. J Chem Eng Data 55:5083–5090
Gupta VK, Mittal A, Gajbe V, Mittal J (2008a) Adsorption of basic fuchsin using waste materials—bottom ash and deoiled soya as adsorbents. J Coll Interf Sci 319:30–39
Gupta VK, Mittal A, Kurup L, Mittal J (2008b) Process development for removal and recovery of Metanil Yellow by adsorption on waste materials—bottom ash and de-oiled soya. J Hazard Mater 151:834–845
Gupta VK, Rastogi A (2008) Equilibrium and kinetic modelling of cadmium(II) biosorption by nonliving algal biomass Oedogonium sp. from aqueous phase. J Hazard Mater 153:759–766
Gupta VK, Rastogi A (2009) Biosorption of hexavalent chromium by raw and acid-treated green alga Oedogonium hatei from aqueous solutions. J Hazard Mater 163:396–402
Gupta VK, Srivastava SK, Tyagi R (2000) Design parameters for the treatment of phenolic wastes by carbon columns (obtained from fertilizer waste material). Water Res 34:1543–1550
Gupta VK, Suhas (2009) Application of low cost adsorbents for dye removal—a review. J Environ Manage 90:2313–2342
Haghseresht F, Lu G (1998) Adsorption characteristics of phenolic compounds onto coal-reject-derived adsorbents. Energy Fuel 12:1100–107
Jain AK, Gupta VK, Sahoo BB, Singh LP (1995) Copper(II)-selective electrodes based on macrocyclic compounds. Anal Proc Incl Anal Commun 32:99–101
Jain AK, Gupta VK, Khurana U, Singh LP (1997) A new membrane sensor for UO +22 ions based on 2-hydroxyacetophenoneoxime–thiourea–trioxane resin. Electroanalysis 9:857–860
Jain AK, Gupta VK, Bhatnagar A, Suhas (2003) A comparative study of adsorbents prepared from industrial wastes for removal of dyes. Sep Sci Technol 38:463–481
Jain R, Sikarwar S (2006) Photocatalytic and adsorption studies on the removal of dye Congo red from wastewater. Int J Environ Pollut 27:158–178
Jain R, Mathur M, Sikarwar S, Mittal A (2007) Removal of hazardous dye Rhodamine B through photocatalytic and adsorption treatments. J Environ Manage 85:956–964
Jain R, Shrivastava M (2008) Adsorptive studies of hazardous dye Tropaeoline 000 from an aqueous phase on to coconut-husk. J Hazard Mater 158:549–556
Jain R, Sikarwar S (2008) Removal of hazardous dye Congo red from waste material. J Hazard Mater 52:942–948
Jain R, Sikarwar S (2009) Adsorptive removal of erythrosine dye onto activated low cost de-oiled mustard. J Hazard Mater 167:627–633
Jain R, Sikarwar S (2010) Adsorptive and desorption studies on toxic dye Erioglaucine over deoiled mustard. J Disper Sci Technol 31:883–893
Jain R, Gupta VK, Sikarwar S (2010) Adsorption and desorption studies on hazardous dye Naphthol Yellow S. J Hazard Mater 18:749–756
Khorsand A, Fazaeli R, Manoochehri M (2010) Role of activated carbon from natural adsorbent for removal of textile dyes: effect of pH, kinetic and adsorbent mass. J Phys Theor Chem 7:173–179
Langergren S, Svenska BK (1898) Zur theorie der sogenannten adsorption geloester stoffe. Veternskapsakad Handlingar 24:1–39
Langmuir I (1918) Adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Mittal A, Gupta VK, Malviya A, Mittal J (2008) Process development for the batch and bulk removal and recovery of a hazardous, water-soluble azo dye Metanil Yellow by adsorption over waste materials (bottom ash and de-oiled soya). J Hazard Mater 151:821–832
Mittal A, Kurup L, Mittal J (2007) Freundlich and Langmuir adsorption isotherms and kinetics for the removal of Tartrazine from aqueous solutions using hen feathers. J Hazard Mater 146:243–248
Preethi S, Sivasamy A, Sivanesan S, Ramamurthi V, Swaminathan G (2006) Removal of safranin basic dye from aqueous solutions by adsorption onto corncob activated carbon. J Ind Eng Chem Res 45:7627–763
Slejko FL (1985) Adsorption technology: a step by step approach to process evaluation application. Marcel Dekker, New York
Srivastava SK, Gupta VK, Dwivedi MK, Jain S (1995) Caesium PVC-crown (dibenzo-24-crown-8) based membrane sensor. Anal Proc Incl Anal Commun 32:21–23
Temkin MI, Pyzhev V (1940) Kinetics of ammonia synthesis on promoted iron catalyst. Acta Physiochimica USSR 12:327–356
Unuabonah EI, Adebowale KO, Olu-Owolabi BI (2007) Kinetic and thermodynamic studies of the adsorption of lead (II) ions onto phosphate-modified kaolinite. J Hazard Mater 144:386–95
Weber TW, Chakkravorti RK (1974) Pore and solid diffusion models for fixed-bed adsorbers. AlCHE Journal 20:228–238
Wu FC, Tseng RL, Juang RS (2009) Initial behavior of intraparticle diffusion model used in the description of adsorption kinetics. Chem Eng J 153:1–8
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Vinod Kumar Gupta
Rights and permissions
About this article
Cite this article
Jain, R., Sharma, P. & Sikarwar, S. Kinetics and isotherm analysis of Tropaeoline 000 adsorption onto unsaturated polyester resin (UPR): a non-carbon adsorbent. Environ Sci Pollut Res 20, 1493–1502 (2013). https://doi.org/10.1007/s11356-012-0994-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-012-0994-x