Abstract
Purpose
Augmented central arterial stiffness (CAS) increases cardiovascular risk. CAS can be augmented by physical exercise and sympathetic activation (SYMP) induced by stressful stimuli. Interestingly, sympathetic vasoconstriction triggered by a sympathetic stimulant is augmented immediately after a strenuous half-marathon compared to at rest. This study assessed whether CAS also augments more post- than pre-half-marathon in response to SYMP. Such assessment takes on relevance considering the growing popularity of strenuous, long-distance endurance exercises.
Methods
13 healthy recreational runners (age 46.1 ± 6.5 years; \(V^{\prime}{\text{O}}_{2} \max\) 54.23 ± 9.31 mlO2/min/kg) provided the following measurements prior to and within 10 min following a strenuous half-marathon: beat-by-beat aortic pulse wave velocity (aPWV; index of CAS), mean blood pressure, and heart rate assessment. Measures were performed at rest and during a 2 min handgrip-mediated SYMP. The effects of the half-marathon and SYMP were assessed by two-way repeated-measures ANOVA.
Results
Measurements of the aPWV pre- and post-race were not significantly different (7.5 ± 0.8 vs 7.8 ± 0.8 m/s, p = 0.34; pre- vs post-race). 2 min of SYMP increased the baseline aPWV post-race (7.8 ± 0.8 vs 8.4 ± 0.8, p = 0.003; rest vs SYMP) but not pre-race (7.5 ± 0.8 vs 7.9 ± 0.9, p = 0.21).
Conclusion
The baseline aPWV assessed 7 to 8 min after a strenuous half-marathon is similar to that pre-race in healthy runners. This agrees with previous studies suggesting CAS being at or below resting values > 5 min following completion of aerobic exercises. The same sympathetic stressor augments CAS to a greater extent 8–10 min post-race than pre-race, suggesting a greater post-exercise stiffening of central artery segments triggered by the same task.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The aortic pulse wave velocity (aPWV) is a reliable indicator of aortic stiffness and an independent predictor of cardiovascular mortality [1]. Its evaluation has gained interest in clinical practice as it can be performed quickly and noninvasively with various techniques and devices [2, 3]. Numerous cut-off limits have been suggested to score the cardiovascular risk according to the subjects’ features [4, 5]. This index is of particular relevance for professionals involved in sports medicine, as suggested by the impressive amount of research performed in the last few years on the effects of the sympathetic nervous system on arterial stiffness [6], as well as on the effects of acute and chronic physical exercises on arterial stiffness in healthy and unhealthy individuals [7, 8]. The interest is understanding whether the stiffness of arterial segments reached in the presence of haemodynamic conditions altered by sympathetic activation and physical exercise may have health implications. However, the effects of the interaction between acute sympathetic activation and physical exercise on arterial stiffness have not yet been investigated.
Augmented central arterial stiffness (CAS) increases the cardiac afterload, blood pressure, and pulse pressure, which thereby augments the risk of acute cardiovascular events regardless of the known disease [9,10,11,12,13]. Augmented CAS after exercise increases cardiac work and myocardial oxygen demand [14]. Concurrently, the higher heart rate reduces the diastolic duration which, along with augmented cardiac afterload, can impair coronary perfusion. Augmented CAS after exercise can also promote the formation of embolisms and rupture of atherosclerotic plaques and aneurysms in central arterial segments due to large and abrupt changes in blood pressure, increased circulating blood catecholamines, and vasomotor oscillations [9]. Increased sympathetic outflow to the heart and blood vessels can further enhance the risk for acute cardiovascular events by augmenting CAS [15, 16]. Since baroreceptors are located in central arterial segments, augmented CAS can result in lower artery wall deformation during blood pressure changes. This may lower the baroreceptor stretching and sensitivity [9, 17] and further increase the sympathetic outflow and circulating catecholamine levels [9, 11, 12].
We have previously shown that sympathetic vasoconstriction in response to acute sympathetic activation (SYMP) triggered by the same handgrip exercise may be augmented immediately after a half-marathon race compared to at rest [18]. Indeed, the normal mean vasodilation after a maneuver causing rapid vasodilation of the leg was reduced by SYMP within 15 min after a half-marathon, whereas it was unaltered before the race. As such, augmented post-exercise sympathetic vasoconstriction in response to the same external stimulus may also increase CAS to a greater extent after a half-marathon race compared to pre-race. Such evaluation takes on relevance, since half-marathon races have gained the most popularity out of all long-distance races within the last few years [19]. This study assessed whether the effects of the same external stimulus capable of activating the sympathetic nervous system on the aPWV, as index of CAS, are augmented immediately after a half-marathon compared to before. It is hypothesized that the same external task will increase the baseline aPWV to a greater extent post-race than pre-race.
Methods
Subjects
We assessed the aPWV in 13 competitive runners (3 females and 10 males; age 46.1 ± 6.5 year; \(V^{\prime}{\text{O}}_{2} \max\) 54.23 ± 9.31 mLO2 min−1 kg−1; 277 ± 32 min of running training/week) prior to and within 10 min following a strenuous half-marathon race. This was performed at rest and during acute SYMP induced by handgrip exercise. The sample size was calculated with a priori power analysis (GPower 3.1.9.7; Universität Düsseldorf, Germany) for an F test (ANOVA, repeated measures, within-factors), partial eta squared of 0.18 [18], statistical power (1 − β) of 0.80, level of significance of 0.05, suggesting the need for 12 individuals. Additionally, augmented sympathetic vasoconstriction post-half-marathon compared to at rest in response to handgrip-mediated SYMP was found with a sample size of 11 individuals [18]. Inclusion criteria consisted of the absence of muscle-skeletal, metabolic, and cardiopulmonary disease, and at least 4 year half-marathon racing experience. This last requirement was essential to guarantee that the race was run at maximum effort. Exclusion criteria consisted of a BMI ≥ 25 kg/m2, diabetes mellitus, hypertensive disorders or any metabolic or neuromuscular chronic condition, and use of any drug that alters neural cardiovascular control. Runners were asked to abstain from alcoholic and caffeine-rich beverages and any kind of training during the 24 h leading up to the tests. Data were collected in the Cardiovascular Physiology Lab, School of Sports Science, University of Verona. The environmental temperature was controlled and set at 22 °C. The half-marathon was organized in Verona. The finish line was located approximately 30 m from our Cardiovascular Physiology Lab. This study was directed under the rules outlined in the Declaration of Helsinki. The Internal Review Board of the Department of Neurological, Biomedical, and Movement Sciences of the University of Verona approved all procedures involving human subjects (165,038). Each participant provided written, informed consent prior to assessment.
Aortic stiffness assessment
Participants performed two maximal handgrip (Saehan SH5001, Germany) contractions with their left hand to determine their maximum voluntary contraction (MVC). All participants were right-handed. Each contraction lasted about 3 s and was separated by 4 min of rest. Pre-race aPWV measures were performed 1 h after the MVC assessment and 3 h before the race start. Participants were sitting and prepared with a beat-by-beat finger blood pressure monitoring system (Finapres Medical System BV, The Netherlands) properly calibrated on the second phalanx of the right hand to assess the mean arterial pressure (MAP) and heart rate (HR). The finger photoplethysmographic signal (FPS) of Finapres was collected at 1 kHz with an analog-to-digital converter (ESP32, AZDelivery, Germany) and analyzed to calculate the aPWV (details below). A 1 min baseline FPS recording was collected pre-race to assess the baseline aPWV at rest. Subsequently, subjects completed a 2 min static overhead handgrip exercise at 30% of MVC with their left hand to acutely activate the sympathetic nervous system [18, 20,21,22,23]. Recording of FPS during the handgrip exercise was used to assess the pre-race aPWV during SYMP. The post-race aPWV was assessed within 10 min following completion of the race repeating the procedures performed pre-race. Specifically, the post-race baseline aPWV was assessed from minute 7 to 8 after the race, whereas the post-race aPWV during SYMP was assessed from minute 8 to 10 after the race. The exercising forearm was elevated 50 cm above the heart to augment the sympathetic activation during handgrip exercise compared to the same exercise performed at heart level [20]. Participants were told to attempt the race with their maximum commitment and, upon reaching the finish line, the rate of perceived exertion was assessed with the Borg CR100 scale.
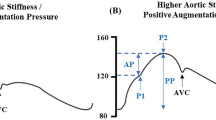
Aortic pulse wave velocity calculation
We employed a novel technique to assess the aPWV by processing the finger photoplethysmographic signal (FPS). The full procedure is described in detail in the paper of Pilt et al. [24]. The FPS was processed via software (MatLab, MathWorks, USA). This method applies the oscillometric working principle developed for the Arteriograph device (TensioMed Kft, Budapest, Hungary) to the FPS. This algorithm determines the time elapsed between the first wave ejected from the left ventricle into the aortic root and its reflection from the aortic bifurcation as the second systolic wave [25] to derive the aortic pulse transit time from the left ventricle outflow tract to the aortic bifurcation. The aortic pulse transit time was determined by calculating the time elapsed from the first zero-crossing point on the first-order FPS derivative to, if visible, the third zero-crossing point on the first-order FPS derivative. If the latter was not visible, the aortic pulse transit time was determined from the first zero-crossing point on the first-order FPS derivative to the second valley of the second-order FPS derivative. The pulse transit distance was measured from the sternal notch to the pubic symphysis. The aPWV was finally calculated as transit distance divided by aortic pulse transit time.
Test–retest aPWV measurement repetitiveness
In a preliminary analysis, a 1 min baseline finger photoplethysmographic signal (FPS) recording was collected after 10 min of rest. Then, a different operator removed and replaced the Finapres cuff on the subjects’ fingers. Another 1 min baseline FPS signal was collected to be compared to the previous one.
Data analysis and statistics
The mean ± SD of aPWV, MAP, and HR were obtained at rest, as well as 1 (SYMP1 min, 45–75 s after SYMP) and 2 min (SYMP2 min, 105–135 s after SYMP) following SYMP. Test-retest repetitiveness was assessed through Bland Altman plots. Post-race Borg CR100 scores were further calculated as mean ± SD and expressed in arbitrary units (AU). Data normality was evaluated by Shapiro–wilk test. The half-marathon and SYMP effects on aPWV, MAP, and HR were assessed by two-way repeated-measures ANOVA (after vs before the race; at rest vs SYMP1 min vs SYMP2 min) with Sidak posthoc test at p < 0.05. GraphPad Prism 8 (GraphPad Software, San Diego, United States) was used for statistical analysis and graphs.
Results
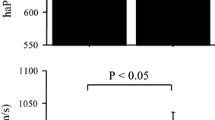
All runners successfully completed the race. The race conclusion time was 96.19 ± 12.41 min. The race day was cloudy with an average temperature of 14.5 °C and a humidity reading between 60 and 79%. Ending values of the BORG CR100 score were 88.52 ± 8.54 AU. Repetitiveness results of the technique are shown in Fig. 1. The bias of the technique was 0.022 m/s, the standard deviation of bias was 0.158 m/s, and the limits of agreement were from −0.29 to 0.33 m/s. ANOVA suggested an effect of the half-marathon (p < 0.036, F > 3.8) and SYMP (p < 0.02, F > 3.5) on the aPWV (p = 0.039, F = 5.3 and p = 0.0025, F = 9.2, respectively) and HR (p < 0.0001, F = 120 and p = 0.014, F = 5.4, respectively). MAP was affected by SYMP (p < 0.0001, F = 8.4) but was not affected by the half-marathon (p = 0.41). ANOVA further suggested SYMP x half-marathon interaction for aPWV (p = 0.0069, F = 6.4), MAP (p < 0.001, F = 16.9) and HR (p < 0.001, F = 14.5).
When comparing post to pre-race, the aPWV was similar at rest and at SYMP1min, but higher at SYMP2min. The pre-race aPWV did not increase during SYMP, whereas the post-race aPWV increased after 2 min of SYMP. The MAP was similar between post and pre-race data at rest, at SYMP1min at SYMP2min and consistently increased during SYMP. The post-race HR was higher at rest, at SYMP1min and at SYMP2min compared to pre-race and consistently increased during SYMP in both before and after the race measurements. Data are reported in Table 1 and Fig. 2.
Discussion
The primary goal of our study was to evaluate whether the baseline aPWV, a surrogate index of the aortic stiffness and CAS, increases to a greater degree post-race than pre-race in response to the same external task capable of activating the sympathetic nervous system. Therefore, it was investigated whether the effects of the half-marathon race change the physiological effects induced by the same external stimulus on the aPWV. We employed a novel technique to measure the aPWV by processing the finger photoplethysmographic signal as previously indicated [24]. This algorithm provides the aortic pulse transit time from the left ventricle to the aortic bifurcation. The aPWV is then calculated as pulse transit distance divided by pulse transit time. This technique displays high repeatability as shown by the small bias of 0.022 m/s we found in our test-retest analysis. Our data show that 2 min of SYMP did not affect the pre-race baseline aPWV but significantly augmented the post-race one. Therefore, the same external stimulus appeared to increase the stiffness of the central arterial segments post-race but not pre-race. The baseline aPWV measured 7 to 8 min after the half-marathon conclusion was statistically similar (p = 0.09) when compared to pre-race resting values. According to the BORG CR100 scale, the overall score of about 88 AU displays a race effort between very and extremely strong.
Pre-exercise resting aPWV
Previous research has questioned whether increased CAS at rest may be a health risk factor [9,10,11,12]. Therefore, cut-off values of pulse wave velocity to score the cardiovascular risk have been proposed [5]. A previous meta-analysis suggested an increase in the relative risk for cardiovascular disease events and mortality by 12% and 9%, respectively, for a chronic increase in cf-PWV of 1 m/s [27]. Chronic endurance training induces anatomical vascular remodeling and changes in neurovascular regulation [28, 29]. It increases the artery diameter, decreases the artery thickness, and changes the artery viscoelastic properties by changing the gene expression of structural proteins [28, 29]. Positive effects of chronic endurance training on vascular health have been shown by increasing artery flow-mediated vasodilation, an important index of coronary artery disease [30]. Regular endurance exercise at low or moderate intensity has also been shown to decrease central arterial stiffness as evidenced by a decrease in cf-PWV [31]. CAS diminishes in individuals who undergo recreational endurance training sessions at low or moderate intensity compared to sedentary individuals, but it can increase again in competitive long-distance athletes due to the higher endurance exercise level [9]. Indeed, strong elevations in systolic arterial pressure (SAP) during physical exercise have been suggested to overload the viscoelastic tissue of the arterial walls and to modify the arterial structure if repeated chronically [32]. Such a condition is also typical of resistance exercise. Indeed, a study found a decreased carotid arterial compliance after chronic resistance training at high intensity, but not at low intensity, which was associated with the acute change in SAP during exercise sessions [33]. The chronic repetition of strenuous endurance exercise sessions has also been shown to contribute to endothelial dysfunction and vascular inflammation, inducing an oxidative vascular environment as a large production of reactive oxygen species overpowers the nitric oxide production [34, 35]. Whether the chronic repetition of strenuous training and races might negatively affect the CAS in competitive endurance athletes has thus been questioned. The pre-race aPWV by about 7.5 m/s we found in our runners is within the limits (6–10 m/s) suggested for healthy individuals between 24 and 62 years old [5]. This suggests that the repetition of strenuous exercises competitive half-marathon runners undergo do not result in out-of-limit augmented CAS. It is relevant to mention that any possible augmented aPWV in competitive endurance runners compared to other populations may not necessarily be linked to increased cardiovascular risk. Competitive runners might present increased resting aPWV values compared to other populations, but be totally healthy. Such a condition could, however, categorize them as individuals at higher cardiovascular risk after preliminary aPWV screening tests.
Effect of a strenuous half-marathon on the aPWV
Our data show that the post-race baseline aPWV assessed 7 to 8 min after the half-marathon was similar to that pre-race. This finding is in agreement with previous studies suggesting CAS be at or below resting values > 5 min after the conclusion of submaximal aerobic exercises [7]. Conversely, resistance exercise has been shown to induce long-lasting CAS increases after the exercise conclusion [8, 13, 33, 36]. Interestingly, our finding reveals an exercise intensity-independent effect on the post-exercise aPWV. This might be due to the similar post- and pre-race MAPs. Indeed, any MAP increase would stretch arterial vessels and increase their stiffness, and vice versa, due to the blood pressure dependence of the stress-strain relationship [37]. In accordance with these findings, previous studies showed that post-exercise decrements of aPWV were associated with lower MAP during the recovery [38].
Effect of SYMP on the aPWV
It was investigated whether the effects of the half-marathon race change the physiological effects on CAS induced by the same external task capable of activating the sympathetic nervous system. The handgrip exercise was shown to reliably increase the muscle sympathetic nerve activity and peripheral resistances as confirmed by the large availability of microneurography recordings [20,21,22]. Raising the exercising forearm above the heart augments the normal sympathetic activation in response to handgrip exercise compared to the same exercise performed at heart level [20]. Consistent with previous investigations [20, 22], SYMP progressively increased MAP ad HR over time before physical exercise. Such responses were also noted after the half-marathon. SYMP did not affect the aPWV before the race. Whether the sympathetic outflow can modulate the stiffness of elastic large arteries at rest has not yet been well defined. Lower body negative pressure mediated SYMP did not alter the compliance of the distal abdominal aorta [15] or carotid artery [16]. However, this result might be due to the central MAP drop needed to unload baroreceptors [37]. In contrast to our results, another study using a similar handgrip exercise to induce SYMP increased the aPWV at rest [39]. However, in that study, the stimulus was applied for twice the time than it had been in ours. This suggests that a similar sympathetic stimulant may increase the CAS at rest but the stressful situation must be longer lasting.
Two minutes of SYMP significantly augmented the post-race baseline aPWV, but did not affect the pre-race baseline aPWV. Therefore, the same external stimulus augmented the baseline stiffness of the central arterial segments post-exercise but not pre-exercise. We have previously shown that peripheral sympathetic vasoconstriction is augmented within 15 min after a half-marathon by specifically using the same handgrip exercise, modality, and timing of stimulus application we employed in the present study [18]. Although defining the underlying mechanisms is beyond the scope of this project, previous studies showed the preeminent role for the sympathetic nervous system in constricting the vascular tissue immediately after endurance exercise than at rest through a mechanism mediated by α1‐adrenergic activity [40, 41]. Furthermore, there might be a synergistic effect of augmented circulating blood catecholamines [42] on the effects of handgrip exercise after a half-marathon. Moreover, the same handgrip workload may correspond to a higher percentage of MVC after the race compared to pre-race due to fatigue [43]. However, the same handgrip exercise intensity was shown to induce less MSNA > 60 min after a prolonged endurance exercise compared to before exercise [44], but MSNA recordings immediately (< 15 min) after prolonged endurance exercise are not available due to methodological limits.
Practical implications
Specialists involved in sports medicine must carefully evaluate the effects of exercises before suggesting them to their athletes, particularly in at-risk individuals [7]. We primarily sought to assess whether the effects of the same external task capable of activating the sympathetic nervous system on the stiffness of central artery segments are augmented after the conclusion on a half-marathon race compared to before the race. A wide variety of stressful stimuli can acutely activate the sympathetic nervous system, including mental stress and emotions [45]. Our data show that the same stressful stimulus augments the baseline stiffness of the central arterial segments post-exercise but not pre-exercise. As mentioned in the introduction, an increase in CAS can augment cardiac work and oxygen consumption [14]. Following the conclusion of physical exercise, this condition occurs in the presence of a reduced diastolic time that may impair coronary perfusion. Unfortunately, there is currently no information to adequately score the impact of a temporary CAS increment on the risk for acute cardiovascular events. However, this finding reveals the need for further investigations into the interaction effects between physical exercise and sympathetic activation on cardiovascular risk. Furthermore, it was unknown if and how CAS changes after a strenuous half-marathon race. This issue is relevant considering that the half-marathon has gained the most popularity out of all long-distance races in terms of number of participants [19]. Our findings reveal that a half-marathon race affects the CAS similarly as aerobic exercises at lower intensities do.
Limitations
The technique we employed to assess the aPWV was the most suitable method for the goals of our study, but not the gold-standard method. The gold-standard cf-PWV assessment requires two different measurements in the carotid and femoral arteries at two separate time points [46]. This method would not allow an appropriate aPWV assessment during SYMP due to the progressive cardiovascular changes over time [22, 47]. Conversely, the method we employed allows the aPWV assessment beat-by-beat and with a single site measurement. Moreover, the method we employed shows high repeatability during test-retest measures compared to other techniques [48]. This study has evaluated absolute values of aPWV reached in the presence of haemodynamics altered by sympathetic activation and physical exercise. The interest was investigating the stiffness of central arterial segments reached in the presence of such haemodynamic conditions. Our study was not intended to investigate how the arterial viscoelastic structure would be in the presence of similar resting haemodynamic conditions, as it is done for other purposes such as evaluating the chronic effects of exercise training or pharmacological therapy on arterial stiffness at rest. In the present study, we specifically used 2 min of handgrip exercise at 30% MVC as sympathetic stimulant. We employed the same methodology we used in our previous study that led to increased sympathetic vasoconstriction after a half-marathon race within 2 min from the stimulus application [18]. The use of other sympathetic stimulants, modalities, or timing of stimulus application is possible but may not guarantee an increased post-race sympathetic vasoconstriction within 2 min from application and the same effects on the aPWV we found in this study [26]. Previous research has shown sex differences in arterial stiffness following running exercise [49]. In this regard, our sample size was not equally divided between males and females. Finally, race-related stress in the pre-race period could have occurred in runners and affected the aPWV. However, we scrupulously followed the aPWV measurement guidelines to obtain the measure in the most basal conditions possible avoiding external interferences.
Conclusions
Two minutes of SYMP significantly augmented the post-race baseline aPWV, but did not affect the pre-race baseline aPWV. Therefore, the same external stimulus augmented the baseline stiffness of the central arterial segments post-exercise but not pre-exercise. The post-race baseline aPWV assessed 7 to 8 min after the half-marathon conclusion was similar to the pre-race baseline aPWV. This is in agreement with previous studies which suggest that CAS be at or below resting values > 5 min following completion of aerobic exercises. This data reveal the need for further investigations into the interaction between endurance exercise and stressful stimuli on cardiovascular risk.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Cavalcante JL, Lima JAC, Redheuil A, Al-Mallah MH (2011) Aortic stiffness: Current understanding and future directions. J Am Coll Cardiol 57:1511–1522
Wilkinson IB, McEniery CM, Schillaci G et al (2010) ARTERY society guidelines for validation of non-invasive haemodynamic measurement devices: Part 1, arterial pulse wave velocity. Artery Res 4:34–40. https://doi.org/10.1016/j.artres.2010.03.001
Jatoi NA, Mahmud A, Bennett K, Feely J (2009) Assessment of arterial stiffness in hypertension: comparison of oscillometric (Arteriograph), piezoelectronic (Complior) and tonometric (SphygmoCor) techniques. J Hypertens 27:2186–2191. https://doi.org/10.1097/HJH.0b013e32833057e8
Mattace-Raso FUS, Hofman A, Verwoert GC et al (2010) Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values.’ Eur Heart J 31:2338–2350. https://doi.org/10.1093/eurheartj/ehq165
Ranjith R, Binu TG, George V et al (2014) Aortic pulse wave velocity and its relationship with complexity of coronary artery disease based on SYNTAX score. Heart Asia 6:109–115. https://doi.org/10.1136/heartasia-2013-010492
Nardone M, Floras JS, Millar PJ (2020) Sympathetic neural modulation of arterial stiffness in humans. Am J Physiol Circ Physiol 319:H1338–H1346. https://doi.org/10.1152/ajpheart.00734.2020
Mutter AF, Cooke AB, Saleh O et al (2017) A systematic review on the effect of acute aerobic exercise on arterial stiffness reveals a differential response in the upper and lower arterial segments. Hypertens Res 40:146–172
García-Mateo P, García-de-Alcaraz A, Rodríguez-Peréz MA, Alcaraz-Ibáñez M (2020) Effects of resistance training on arterial stiffness in healthy people: a systematic review. J Sports Sci Med 19:444
Sardeli AV, Chacon-Mikahil MPT (2016) Is the exercise-induced increase in central arterial stiffness a risk factor for health? J Arch mil Med 4:e36833. https://doi.org/10.5812/jamm.36833
Rognmo O, Moholdt T, Bakken H et al (2012) Cardiovascular risk of high-versus moderate-intensity aerobic exercise in coronary heart disease patients. Circulation 126:1436–1440. https://doi.org/10.1161/CIRCULATIONAHA.112.123117
Thompson PD, Franklin BA, Balady GJ et al (2007) Exercise and acute cardiovascular events: placing the risks into perspective a scientific statement from the american heart association council on nutrition, physical activity, and metabolism and the council on clinical cardiology. Circulation 115:2358–2368
Albert CM, Mittleman MA, Chae CU et al (2000) Triggering of sudden death from cardiac causes by vigorous exertion. N Engl J Med 343:1355–1361. https://doi.org/10.1056/NEJM200011093431902
Yoon ES, Jung SJ, Cheun SK et al (2010) Effects of acute resistance exercise on arterial stiffness in young men. Korean Circ J 40:16–22. https://doi.org/10.4070/kcj.2010.40.1.16
Boyette LC, Manna B (2020) Physiology, Myocardial oxygen demand. Statpearls
Sonesson B, Vernersson E, Hansen F, Länne T (1997) Influence of sympathetic stimulation on the mechanical properties of the aorta in humans. Acta Physiol Scand 159:139–145. https://doi.org/10.1046/j.1365-201X.1997.581343000.x
Pannier B, Slama MA, London GM et al (1995) Carotid arterial hemodynamics in response to LBNP in normal subjects: methodological aspects. J Appl Physiol 79:1546–1555. https://doi.org/10.1152/jappl.1995.79.5.1546
Chesterton LJ, Sigrist MK, Bennett T et al (2005) Reduced baroreflex sensitivity is associated with increased vascular calcification and arterial stiffness. Nephrol Dial Transplant 20:1140–1147. https://doi.org/10.1093/ndt/gfh808
Gentilin A, Tarperi C, Skroce K et al (2021) Effect of acute sympathetic activation on leg vasodilation before and after endurance exercise. J Smooth Muscle Res 57:53–67. https://doi.org/10.1540/JSMR.57
Gómez-Molina J, Ogueta-Alday A, Camara J et al (2017) Predictive variables of half-marathon performance for male runners. J Sport Sci Med 16:187–194
Michikami D, Kamiya A, Fu Q et al (2002) Forearm elevation augments sympathetic activation during handgrip exercise in humans. Clin Sci 103:295–301. https://doi.org/10.1042/cs1030295
Saito M, Mano T, Abe H, Iwase S (1986) Responses in muscle sympathetic nerve activity to sustained hand-grips of different tensions in humans. Eur J Appl Physiol Occup Physiol 55:493–498. https://doi.org/10.1007/BF00421643
Saito M, Mano T, Iwase S (1990) Changes in muscle sympathetic nerve activity and calf blood flow during static handgrip exercise. Eur J Appl Physiol Occup Physiol 60:277–281. https://doi.org/10.1007/BF00379396
Yoshizawa M, Shimizu S, Kagaya A (2006) Changes in brachial and femoral artery vascular conductance in non-exercising limbs during handgrip exercise. Japanese J Phys Fit Sport Med 55:159–161. https://doi.org/10.7600/jspfsm.55.s159
Pilt K, Meigas K, Viigimaa M, Temitski K (2011) Possibility to use finapres signal for the estimation of aortic pulse wave velocity. IFMBE Proc 37:524–527. https://doi.org/10.1007/978-3-642-23508-5_136
Baulmann J, Schillings U, Rickert S et al (2008) A new oscillometric method for assessment of arterial stiffness: comparison with tonometric and piezo-electronic methods. J Hypertens 26:523–528. https://doi.org/10.1097/HJH.0b013e3282f314f7
Dyson KS, Shoemaker JK, Hughson RL (2006) Effect of acute sympathetic nervous system activation on flow-mediated dilation of brachial artery. Am J Physiol - Hear Circ Physiol 290:H1446–H1453. https://doi.org/10.1152/ajpheart.00771.2005
Zhong Q, Hu M, Cui Y et al (2018) Carotid-femoral pulse wave velocity in the prediction of cardiovascular events and mortality: an updated systematic review and meta-analysis. Angiology 69:617–629. https://doi.org/10.1177/0003319717742544
Green DJ, Hopman MTE, Padilla J et al (2017) Vascular adaptation to exercise in humans: role of hemodynamic stimuli. Physiol Rev 97:495–528. https://doi.org/10.1152/physrev.00014.2016
Green DJ, Spence A, Rowley N et al (2012) Vascular adaptation in athletes is there an “athlete’s artery”? Exp Physiol 97:295–304. https://doi.org/10.1113/expphysiol.2011.058826
Dawson EA, Green DJ, Timothy Cable N, Thijssen DHJ (2013) Effects of acute exercise on flow-mediated dilatation in healthy humans. J Appl Physiol 115:1589–1598. https://doi.org/10.1152/japplphysiol.00450.2013
Huang C, Wang J, Deng S et al (2016) The effects of aerobic endurance exercise on pulse wave velocity and intima media thickness in adults: a systematic review and meta-analysis. Scand J Med Sci Sports 26:478–487. https://doi.org/10.1111/SMS.12495
Dobrin PB (1995) Mechanical factors associated with the development of intimal and medial thickening in vein grafts subjected to arterial pressure: a model of arteries exposed to hypertension. Hypertension 26:38–43. https://doi.org/10.1161/01.HYP.26.1.38
Ozaki H, Yasuda T, Ogasawara R et al (2013) Effects of high-intensity and blood flow-restricted low-intensity resistance training on carotid arterial compliance: role of blood pressure during training sessions. Eur J Appl Physiol 113:167–174. https://doi.org/10.1007/s00421-012-2422-9
Durand MJ, Gutterman DD (2014) Exercise and vascular function: How much is too much? Can J Physiol Pharmacol 92:551–557
Bergholm R, Mäkimattila S, Valkonen M et al (1999) Intense physical training decreases circulating antioxidants and endothelium-dependent vasodilatation in vivo. Atherosclerosis 145:341–349. https://doi.org/10.1016/S0021-9150(99)00089-1
Pierce DR, Doma K, Raiff H et al (2018) Influence of exercise mode on post-exercise arterial stiffness and pressure wave measures in healthy adult males. Front Physiol 9(9):1468. https://doi.org/10.3389/FPHYS.2018.01468
Nichols W, O’Rourke M, Vlachopoulos C (2011) McDonald’s blood flow in arteries: theoretical, experimental and clinical principles, 6th editio. CRC Press, London
Milatz F, Ketelhut S, Ketelhut RG (2015) Favorable effect of aerobic exercise on arterial pressure and aortic pulse wave velocity during stress testing. Vasa Eur J Vasc Med 44:271–276. https://doi.org/10.1024/0301-1526/a000441
Mäki-Petäjä KM, Barrett SML, Evans SV et al (2016) The role of the autonomic nervous system in the regulation of aortic stiffness. Hypertension 68:1290–1297. https://doi.org/10.1161/HYPERTENSIONAHA.116.08035
Atkinson CL, Lewis NCS, Carter HH et al (2015) Impact of sympathetic nervous system activity on post-exercise flow-mediated dilatation in humans. J Physiol 593:5145–5156. https://doi.org/10.1113/JP270946
Tymko MM, Tremblay JC, Hansen AB et al (2017) The effect of α1-adrenergic blockade on post-exercise brachial artery flow-mediated dilatation at sea level and high altitude. J Physiol 595:1671–1686. https://doi.org/10.1113/JP273183
Danese E, Tarperi C, Salvagno GL et al (2018) Sympatho-adrenergic activation by endurance exercise. Effect on metanephrines spillover and its role in predicting athlete’s performance. Oncotarget 9:15650–15657. https://doi.org/10.18632/oncotarget.24584
Taylor JL, Gandevia SC (2008) A comparison of central aspects of fatigue in submaximal and maximal voluntary contractions. J Appl Physiol 104:542–550. https://doi.org/10.1152/JAPPLPHYSIOL.01053.2007
Halliwill JR, Taylor JA, Eckberg DL (1996) Impaired sympathetic vascular regulation in humans after acute dynamic exercise. J Physiol 495:279–288. https://doi.org/10.1113/jphysiol.1996.sp021592
Fontes MA, Xavier CH, Marins FR et al (2014) Emotional stress and sympathetic activity: contribution of dorsomedial hypothalamus to cardiac arrhythmias. Brain Res 1554:49–58. https://doi.org/10.1016/J.BRAINRES.2014.01.043
Laurent S, Cockcroft J, Van Bortel L et al (2006) Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 27:2588–2605
Jacobsen TN, Hansen J, Nielsen HV et al (1994) Skeletal muscle vascular responses in human limbs to isometric handgrip. Eur J Appl Physiol Occup Physiol 69:147–153. https://doi.org/10.1007/BF00609407
Parati G, De Buyzere M (2010) Evaluating aortic stiffness through an arm cuff oscillometric device: is validation against invasive measurements enough? J Hypertens 28:2003–2006
Nieman D, Dew D, Krasen P (2013) Gender difference in the acute influence of a 2-hour run on arterial stiffness in trained runners. Res Sports Med 21:66–77. https://doi.org/10.1080/15438627.2012.738445
Acknowledgements
The authors thank Anna Vander Veen and Rhea Gupta for their contribution to the manuscript formatting.
Funding
Open access funding provided by Università degli Studi di Verona within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
Study concept and design (AG); half-marathon organization (CT; KS); data acquisition and analysis (AG); original draft preparation (AG); review and critical revision of the manuscript (AG; CT; KS; AC; FS); all authors approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Ethical approval and Informed consent
The Internal Review Board of the Department of Neurological, Biomedical, and Movement Sciences of the University of Verona approved all procedures involving human subjects (prot. n. 165038). Each participant provided written, informed consent prior to assessment.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised to add missing OASIS funding note.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visithttp://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gentilin, A., Tarperi, C., Skroce, K. et al. Effects of acute sympathetic activation on the central artery stiffness after strenuous endurance exercise. Sport Sci Health 18, 1439–1447 (2022). https://doi.org/10.1007/s11332-022-00941-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11332-022-00941-0