Abstract
Purpose
Sleep apnea-specific hypoxic burden (SASHB) is a polysomnographic metric that comprehensively measures the degree of nocturnal desaturation caused by obstructive sleep apnea. This research was conducted to elucidate the relationship between SASHB and coronary artery disease (CAD) severity.
Methods
We carried out a prospective study of hospitalized patients with CAD of unstable angina who were expected to undergo invasive coronary angiography at Beijing Anzhen Hospital from February to September 2023. SASHB values were calculated using a self-programmed C + + program. Multivariable logistic regression analysis was applied to identify the association between SASHB and the prevalence of severe CAD, documented by the Gensini Score, and the SYNTAX (Synergy between Percutaneous Coronary Intervention With Taxus and Cardiac Surgery) Score.
Results
This study enrolled 137 patients with a median age of 59 years, 96 (70.1%) of whom were male. A total of 125 (91.2%) patients had coronary stenosis of ≥ 50% in at least one location. Patients with a high SASHB of ≥ 18% min/h had a significantly higher Gensini Score (32.0 vs. 18.5, P = 0.002) and SYNTAX Score (14.0 vs. 7.0, P = 0.002) than those with a low SASHB. After adjusting for multiple covariates, a high SASHB was significantly associated with the prevalence of severe CAD, determined by a Gensini Score ≥ 21 (OR 2.67, P = 0.008) or a SYNTAX Score > 22 (OR 4.03, P = 0.016).
Conclusion
Our findings revealed a robust and independent association between SASHB and CAD severity in patients with unstable angina, highlighting the potential value of SASHB as a predictor of risk and a target for interventions aimed at preventing cardiovascular diseases.
Trial registration
Chinese Clinical Trial Registry No. ChiCTR2300067991 on February 2, 2023.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea (OSA), characterized by recurrent interruptions in respiration with intermittent hypoxemia during sleep, is broadly acknowledged as a contributing factor to coronary artery disease (CAD). The apnea–hypopnea index (AHI) is generated using polysomnography to describe the frequency of partial (hypopnea) and complete (apnea) breathing interruptions, allowing the determination of different degrees of OSA severity according to the number of these events. However, no significant association has been found between AHI and the severity of coronary artery lesions [1,2,3]. Moreover, efforts to treat OSA based on the evaluation and reduction of AHI failed to reduce the incidence of major cardiac events in large clinical trials [4, 5]. Therefore, AHI, as a measure of OSA severity, has inherent limitations and does not fully capture the cardiovascular risk associated with OSA [6]. Consequently, developing more sensitive clinical biomarkers that better reflect the close association between OSA and CAD is essential.
Hypoxia is considered the major pathogenic factor underlying OSA [7, 8]. Therefore, hypoxic indices are more powerful predictors of cardiovascular outcomes than AHI [9,10,11,12]. The sleep apnea-specific hypoxic burden (SASHB) is a score that accounts for the duration, severity, and frequency of respiratory-related desaturation events [10, 11, 13]. SASHB has been shown to be more sensitive than AHI in predicting incident heart failure and cardiovascular disease-related mortality among participants in the Sleep Heart Health Study [10] and the Osteoporotic Fractures in Men Study [11]. Although SASHB appears to be one of the most promising indices for predicting cardiovascular risk, direct evidence of the association between SASHB and structural impairment in coronary disease remains lacking. This study used the innovative algorithm of Azarbarzin et al. [11] to calculate SASHB and comprehensively evaluated its potential association with CAD severity, determined by invasive angiography.
Study design and methods
Study population
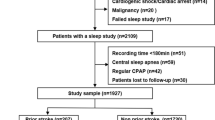
We enrolled individuals diagnosed with unstable angina who were hospitalized at Beijing Anzhen Hospital and scheduled for coronary angiography (and possibly subsequent percutaneous coronary intervention) from February to September 2023.The inclusion criteria were as follows: (1) availability of medical staff and research resources and (2) stable vital signs enabling overnight polysomnography. We excluded patients who met any of the following criteria: (1) acute myocardial infarction verified by cardiac enzymes and electrocardiography; (2) previous revascularization, i.e., percutaneous transluminal coronary angioplasty, percutaneous coronary intervention, or coronary artery bypass grafting; (3) severe insomnia with an Insomnia Severity Index of ≥ 22; (4) end-stage chronic diseases, such as heart failure of the New York Heart Association stage IV, advanced cancer, and renal failure on dialysis; (5) cognitive disorders or acute psychiatric episodes; (6) previous treatment with positive airway pressure for OSA or other disorders within the last 6 months; and (7) use of nocturnal oxygen supplementation that could not be withheld during sleep test. This study was approved by the Institutional Review Board of Beijing Anzhen Hospital, with each patient providing written informed consent.
Overnight polysomnography
Unattended overnight polysomnography was performed using the Alice PDx portable sleep diagnostic system, manufactured by Philips Respironics, located in Murrysville, PA, USA. Bedtime was set between 10:00 PM and 6:00 AM on the night prior to coronary angiography. Electroencephalography, electromyography, electrooculography, electrocardiography, airflow through nasal pressure and thermistor, snore microphone, pulse oximetry, and thoracic-abdominal respiratory inductance plethysmography were performed. Registered polysomnographic technologists scored the sleep stages, respiratory events, desaturation events, and arousals. Apnea was noted when a ≥ 90% reduction in airflow occurred for at least 10 s, while hypopnea referred to a ≥ 30% decrease in airflow for at least 10 s with a ≥ 3% desaturation or microarousal [14]. The total number of both apnea and hypopnea incidents was divided by the sleep duration to calculate AHI, and an AHI of ≥ 15 events/h was indicative of OSA. The oxygen desaturation index (ODI) was defined as the hourly frequency of the pulsus oxygen saturation drop of ≥ 3% from baseline. The mean oxygen saturation during sleep (meanSpO2), lowest oxygen saturation during sleep (minSpO2), and percentage of sleep time with oxygen saturation < 90% (T90SpO2) were directly derived from a polysomnography report. The Epworth Sleepiness Scale, Insomnia Severity Index, Zung Self-rating Anxiety Scale, Zung Self-rating Depression Scale, and Berlin Questionnaire were administered before polysomnography [15,16,17,18]. Morning blood pressure and heart rate were measured while sitting for 10 min after getting up from bed.
SASHB calculation
The SASHB value was determined using the C + + program (compiled in Microsoft Visual Studio Premium 2012, version 11.0.50727.1 RTMREL) based on the innovative algorithm of Azarbarzin et al. [11]. This technique involved computing the area under each respiratory event-related oxygen desaturation curve, considering both the time span and depth of each desaturation event. The beginning and end points of each curve were identified using a search window. This window was defined by aligning all the curves of respiratory event-related oxygen desaturation with the endpoint of each respiratory event marked as time 0. The area under each respiratory-related desaturation curve within the specific search window was then summed and divided by the overall sleep duration (measured in hours) to obtain the SASHB value.
Assessment of coronary artery stenosis
Invasive coronary angiography with iodine contrast was performed to detect coronary stenosis and occlusion. Without access to polysomnographic data, two certified cardiologists independently calculated both the Gensini Score and SYNTAX (Synergy between Percutaneous Coronary Intervention With Taxus and Cardiac Surgery) Score. These scores assess both the severity of coronary lesions and the significance of affected areas in the coronary circulation. The Gensini Score was calculated manually following an available algorithm [19], while the SYNTAX Score was determined using an online calculator [20]. Higher scores indicate more severe coronary lesions in both evaluation systems.
Statistical analysis
Continuous variables were reported using the median along with the interquartile range (25th and 75th percentiles) owing to their skewed distributions, as observed using the Shapiro-Wilk test for normality. Dichotomous variables were reported as frequencies (percentages). Wilcoxon tests were used to compare continuous data, while Pearson’s χ2 tests were applied for the comparison of dichotomous data. To further investigate the effect of disordered breathing on CAD, we used a median split to group participants based on the median values of SASHB (18% min/h), meanSpO2 (94%), and T90SpO2 (0.6% rounded to 1%), together with the conventional cut-offs of ODI (15 events/h) and minSpO2 (85%). Patients with a Gensini Score or a SYNTAX Score above the median value were classified as having severe CAD. Furthermore, a SYNTAX Score of > 22, which is a conventional criterion [21], was also used to define severe CAD. A multivariate logistic regression analysis was performed to investigate the associations between sleep indices and severe CAD. Model 1 adjusted for age and sex, while Model 2 further incorporated the major risk factors for CAD, such as smoking status, hypertension, hemoglobin A1c, and low-density lipoprotein cholesterol (LDL) [22]. To investigate the potential dose–response relationship between SASHB and CAD severity, multiple linear regression analysis was conducted, incorporating significant variables identified in univariate regression model (log SASHB and hemoglobin A1c), major demographic variables (age and sex), and widely recognized risk factors for CAD (smoking, hypertension, and LDL). SASHB underwent log-transformation due to its skewed distribution in the regression analysis. Data analyses were performed with JMP version 16, and statistical significance was defined by a two-sided P-value of < 0.05.
Results
Basic characteristics of study patients
This study included 137 patients with a median age of 59 years, of whom 96 (70.1%) were male. Among these patients, 71 (51.8%) were diagnosed with OSA by polysomnography. Furthermore, 72 (52.6%) of the patients underwent percutaneous coronary intervention, while 16 (11.7%) only had percutaneous transluminal coronary angioplasty, and 10 (7.3%) were referred for coronary artery bypass grafting after angiography. Twelve (8.8%) patients had arterial stenosis of < 50% in the coronary tree. Despite the OSA group having a higher body mass index, neck circumference, and waist circumference, the demographic and clinical data were comparable between the OSA and non-OSA groups, as detailed in Table 1.
Table 2 presents the sleep variables of the study patients categorized according to the diagnosis of OSA. Patients with OSA had heart rate and morning blood pressure comparable to those of the non-OSA population. Most of the patients had a low prevalence of somnolence, insomnia, anxiety, and depression. The Berlin Questionnaire accurately identified 46 (64.8%) patients with OSA before polysomnography.
Sleep apnea and distribution of coronary lesions
Patients with OSA had a higher occurrence of ≥ 50% stenosis in the right coronary artery than those without OSA (62.0% vs. 36.4%, P = 0.003). Similarly, the prevalence of ≥ 50% stenosis in the right coronary artery was greater in patients with higher values of SASHB than those with lower SASHB values (63.8% vs. 35.3%, P < 0.001) (Table 3). However, the investigation revealed no significant differences in the distribution of ≥ 50% stenosis in the left main-left anterior descending branch and left circumflex branch between the studied sub-groups.
Sleep disorder and CAD severity
Patients with OSA had more severe coronary lesions than those without OSA, determined by the Gensini Score (28.0 vs. 20.0, P = 0.020) and SYNTAX score (13.0 vs. 8.0, P = 0.021) (Table 4). Patients with high SASHB had more severe coronary lesions than those with low SASHB (Gensini Score: 32.0 vs. 18.5, P = 0.002; SYNTAX Score: 14.0 vs. 7.0, P = 0.002). Similarly, patients with a higher ODI, lower minSpO2, and higher T90SpO2 exhibited a higher Gensini Score and SYNTAX Score than their counterparts.
Association of SASHB with the prevalence of severe CAD
A significant association was shown between high SASHB levels and the prevalence of severe CAD, defined by the Gensini Score and SYNTAX Score greater than their respective median values of 21 and 11 (OR 2.51, 95% CI 1.26 − 5.00, P = 0.009 and OR 3.23, 95% CI 1.60 − 6.50, P = 0.001) (Table 5). This association persisted after adjusting for demographic factors for age and sex in Model 1 (OR 2.56, 95% CI 1.28 − 5.13, P = 0.008 and OR 3.17, 95% CI 1.56 − 6.47, P = 0.002), and for six major risk factors for CAD (age, sex, smoking, hypertension, hemoglobin A1c, and LDL) in Model 2 (OR 2.67, 95% CI 1.30 − 5.48, P = 0.008 and OR 3.83, 95% CI 1.77 − 8.30, P < 0.001). When modeling SASHB as a continuous variable, an increase of 1-U in SASHB led to approximately 2% higher odds of having severe CAD in univariate (OR 1.02, 95% CI 1.01 − 1.03, P = 0.006) and in adjusted Model 1 and Model 2 (OR 1.02, 95% CI 1.00 − 1.03, P = 0.007 and OR 1.02, 95% CI 1.00 − 1.03, P = 0.014). Furthermore, when stratifying patients according to the conventional criterion of severe CAD (SYNTAX Score > 22), patients with high SASHB also showed higher odds of having severe CAD in adjusted Model 2 (OR 4.03, 95% CI 1.30 − 12.47, P = 0.016).
The multiple linear regression analysis revealed a significant association between log SASHB and the values of Gensini Score (β = 5.99, P = 0.001) and SYNTAX Score (β = 1.90, P = 0.005), with age, sex, hemoglobin A1c, smoking, hypertension, and LDL all incorporated as variables in the model (Table 6).
Discussion
This study provides evidence of a robust correlation between SASHB levels and the structural severity of CAD in individuals subjected to invasive coronary angiography. Our findings highlight the need to accelerate the application of SASHB in cardiovascular community.
Our results are consistent with those of previous studies demonstrating that SASHB is a more sensitive indicator than AHI for predicting adverse cardiovascular outcomes [10, 11]. Although AHI is a universally accepted metric for stratifying the severity of OSA, it has inherent limitations and does not reflect several key pathophysiological mechanisms activated by aberrant respiratory events during sleep. For example, AHI does not account for the duration of respiratory events or the severity of subsequent episodes of desaturation. By analyzing the frequency, duration, and depth of respiratory-related desaturation, the SASHB proved to be a robust tool for assessing OSA severity. Additional investigations should be undertaken to examine the link between SASHB and other previously identified OSA phenotypes, such as low arousal threshold, excessive sleepiness, and rapid eye movement-dominant OSA subtypes. In addition to CAD, it is important to validate the predictive value of SASHB in other cardiovascular diseases, such as hypertension, atrial fibrillation, and aortic dissection. Although SASHB has its merits, it is not without limitations. For example, SASHB does not consider an individual’s ability to endure hypoxia, as some patients may tolerate hypoxia better than others. Therefore, it is advisable to interpret the results of the SASHB in conjunction with other clinical data to obtain a more comprehensive understanding of an individual’s sleep.
To our knowledge, this study represents the first attempt to elucidate the links between SASHB levels and coronary structural impairment as measured by the Gensini Score and SYNTAX Score, both of which are prognostically relevant algorithms [23,24,25]. However, based on these cross-sectional results, we cannot directly conclude that SASHB is associated with cardiovascular outcomes, which has been partially confirmed in other studies on heart failure and cardiovascular fatality [10, 11]. Longitudinal studies are warranted to observe the development of coronary lesions in patients with high SASHB levels and other OSA phenotypes.
Computation of SASHB based on current polysomnography remains challenging and presently no standardized operating procedure is available. Azarbarzin et al. devised a unique search window for each subject, facilitating the computation of the area under the desaturation curve, even in cases where desaturation did not show an unambiguous beginning and end. Although the subject-specific search window strategy is convenient and practical, it has certain limitations. For example, long desaturation events may extend the search window, leading to the omission of SASHB values outside the window. In contrast, spontaneous desaturation not caused by respiratory events may still be included if they fall within the window, resulting in an overestimation of the SASHB values. Therefore, more refined techniques are required to accurately calculate the SASHB value and fulfill its promising clinical potential.
Strengths and limitations
Direct assessment of the severity of CAD using angiography is a strength of our research. However, the study design had certain limitations. All participants were hospitalized for angina; thus, the clinical value of the SASHB could not be extrapolated to the general population. Notably, a cohort study that recruited community residents for osteoporosis research concluded that SASHB had cardiovascular prognostic significance [11]. Second, the cross-sectional study design precluded establishing a temporal association between SASHB and the development of severe CAD. A causal relationship would require confirmation through repeated coronary angiography during the follow-up assessment.
Conclusion
Our study findings reveal that individuals with unstable angina who have high SASHB levels may show more severe coronary lesions, as observed by invasive angiography. Further studies are necessary to verify the detrimental effects of high SASHB levels on the development of CAD. Additionally, improvements in the algorithm used to calculate SASHB scores may facilitate their clinical utility.
Data availability
The datasets created and/or analyzed in this study are accessible from the corresponding author.
References
Rivera-Perez SJ, Martinez D, Araujo GN, Goncalves SC, Lazzaretti LK, Wainstein RV, Wainstein MV, Ribeiro JP (2019) Severity of obstructive sleep apnea and extension of coronary artery disease. Sleep Breath 23(3):747–752. https://doi.org/10.1007/s11325-018-1769-5
Newman SB, Kundel V, Matsuzaki M, Reid M, Kizer JR, Kaplan RC, Fayad ZA, Mani V, Shea S, Allison M, Criqui MH, Lutsey PL, McClelland RL, Redline S, Shah NA (2021) Sleep apnea, coronary artery calcium density, and cardiovascular events: results from the Multi-Ethnic Study of Atherosclerosis. J Clin Sleep Med 17(10):2075–2083. https://doi.org/10.5664/jcsm.9356
Zeng Y, Yang S, Wang X, Fan J, Nie S, Wei Y (2019) Prognostic impact of residual SYNTAX score in patients with obstructive sleep apnea and acute coronary syndrome: a prospective cohort study. Respir Res 20(1):43. https://doi.org/10.1186/s12931-019-1008-z
McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, Mediano O, Chen R, Drager LF, Liu Z, Chen G, Du B, McArdle N, Mukherjee S, Tripathi M, Billot L, Li Q, Lorenzi-Filho G, Barbe F, Redline S, Wang J, Arima H, Neal B, White DP, Grunstein RR, Zhong N, Anderson CS, Investigators S, Coordinators (2016) CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med 375(10):919–931. https://doi.org/10.1056/NEJMoa1606599
Sanchez-de-la-Torre M, Sanchez-de-la-Torre A, Bertran S, Abad J, Duran-Cantolla J, Cabriada V, Mediano O, Masdeu MJ, Alonso ML, Masa JF, Barcelo A, de la Pena M, Mayos M, Coloma R, Montserrat JM, Chiner E, Perello S, Rubinos G, Minguez O, Pascual L, Cortijo A, Martinez D, Aldoma A, Dalmases M, McEvoy RD, Barbe F, Spanish Sleep N (2020) Effect of obstructive sleep apnoea and its treatment with continuous positive airway pressure on the prevalence of cardiovascular events in patients with acute coronary syndrome (ISAACC study): a randomised controlled trial. Lancet Respir Med 8(4):359–367. https://doi.org/10.1016/S2213-2600(19)30271-1
Heinzer R, Eckert D (2020) Treatment for obstructive sleep apnoea and cardiovascular diseases: are we aiming at the wrong target? Lancet Respir Med 8(4):323–325. https://doi.org/10.1016/S2213-2600(19)30351-0
Lavie L (2015) Oxidative stress in obstructive sleep apnea and intermittent hypoxia—revisited—the bad ugly and good: implications to the heart and brain. Sleep Med Rev 20:27–45. https://doi.org/10.1016/j.smrv.2014.07.003
Dewan NA, Nieto FJ, Somers VK (2015) Intermittent hypoxemia and OSA: implications for comorbidities. Chest 147(1):266–274. https://doi.org/10.1378/chest.14-0500
Tan BKJ, Teo YH, Tan NKW, Yap DWT, Sundar R, Lee CH, See A, Toh ST (2022) Association of obstructive sleep apnea and nocturnal hypoxemia with all-cancer incidence and mortality: a systematic review and meta-analysis. J Clin Sleep Med 18(5):1427–1440. https://doi.org/10.5664/jcsm.9772
Azarbarzin A, Sands SA, Taranto-Montemurro L, Vena D, Sofer T, Kim SW, Stone KL, White DP, Wellman A, Redline S (2020) The sleep apnea-specific hypoxic burden predicts incident heart failure. Chest 158(2):739–750. https://doi.org/10.1016/j.chest.2020.03.053
Azarbarzin A, Sands SA, Stone KL, Taranto-Montemurro L, Messineo L, Terrill PI, Ancoli-Israel S, Ensrud K, Purcell S, White DP, Redline S, Wellman A (2019) The hypoxic burden of sleep apnoea predicts cardiovascular disease-related mortality: the Osteoporotic Fractures in Men Study and the Sleep Heart Health Study. Eur Heart J 40(14):1149–1157. https://doi.org/10.1093/eurheartj/ehy624
Xie J, Sert Kuniyoshi FH, Covassin N, Singh P, Gami AS, Wang S, Chahal CA, Wei Y, Somers VK (2016) Nocturnal hypoxemia due to obstructive sleep apnea is an independent predictor of poor prognosis after myocardial infarction. J Am Heart Assoc 5(8). https://doi.org/10.1161/JAHA.115.003162
Blanchard M, Gerves-Pinquie C, Feuilloy M, Le Vaillant M, Trzepizur W, Meslier N, Goupil F, Pigeanne T, Balusson F, Oger E, Sabil A, Girault JM, Gagnadoux F, group Es (2021) Hypoxic burden and heart rate variability predict stroke incidence in sleep apnoea. Eur Respir J 57(3). https://doi.org/10.1183/13993003.04022-2020
Troester M, Quan S, Berry R (2023) The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. https://aasm.org/clinical-resources/scoring-manual/. Accessed 1 Mar 2023
Goncalves MT, Malafaia S, Moutinho Dos Santos J, Roth T, Marques DR (2023) Epworth sleepiness scale: a meta-analytic study on the internal consistency. Sleep Med 109:261–269. https://doi.org/10.1016/j.sleep.2023.07.008
Duan X, Zheng M, Zhao W, Huang J, Lao L, Li H, Lu J, Chen W, Liu X, Deng H (2022) Associations of depression, anxiety, and life events with the risk of obstructive sleep apnea evaluated by Berlin questionnaire. Front Med (Lausanne) 9:799792. https://doi.org/10.3389/fmed.2022.799792
Jokelainen J, Timonen M, Keinanen-Kiukaanniemi S, Harkonen P, Jurvelin H, Suija K (2019) Validation of the Zung self-rating depression scale (SDS) in older adults. Scand J Prim Health Care 37(3):353–357. https://doi.org/10.1080/02813432.2019.1639923
Morin CM, Belleville G, Belanger L, Ivers H (2011) The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep 34(5):601–608. https://doi.org/10.1093/sleep/34.5.601
Yokokawa T, Yoshihisa A, Kiko T, Shimizu T, Misaka T, Yamaki T, Kunii H, Nakazato K, Ishida T, Takeishi Y (2020) Residual Gensini score is associated with long-term cardiac mortality in patients with heart failure after percutaneous coronary intervention. Circ Rep 2(2):89–94. https://doi.org/10.1253/circrep.CR-19-0121
Gameren Mv. the SYNTAX Score. 2022; https://syntaxscore.org/. Accessed January 1st, 2023
Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ, Stahle E, Feldman TE, van den Brand M, Bass EJ, Van Dyck N, Leadley K, Dawkins KD, Mohr FW, Investigators S (2009) Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 360(10):961–972. https://doi.org/10.1056/NEJMoa0804626
Malakar AK, Choudhury D, Halder B, Paul P, Uddin A, Chakraborty S (2019) A review on coronary artery disease, its risk factors, and therapeutics. J Cell Physiol 234(10):16812–16823. https://doi.org/10.1002/jcp.28350
Garg S, Sarno G, Garcia-Garcia HM, Girasis C, Wykrzykowska J, Dawkins KD, Serruys PW, Investigators A-I (2010) A new tool for the risk stratification of patients with complex coronary artery disease: the Clinical SYNTAX Score. Circ Cardiovasc Interv 3(4):317–326. https://doi.org/10.1161/CIRCINTERVENTIONS.109.914051
Girasis C, Garg S, Raber L, Sarno G, Morel MA, Garcia-Garcia HM, Luscher TF, Serruys PW, Windecker S (2011) SYNTAX score and Clinical SYNTAX score as predictors of very long-term clinical outcomes in patients undergoing percutaneous coronary interventions: a substudy of SIRolimus-eluting stent compared with pacliTAXel-eluting stent for coronary revascularization (SIRTAX) trial. Eur Heart J 32(24):3115–3127. https://doi.org/10.1093/eurheartj/ehr369
Wang KY, Zheng YY, Wu TT, Ma YT, Xie X (2021) Predictive value of Gensini score in the long-term outcomes of patients with coronary artery disease who underwent PCI. Front Cardiovasc Med 8:778615. https://doi.org/10.3389/fcvm.2021.778615
Funding
This research was financially supported by the National Natural Science Foundation of People’s Republic of China, through grants 81970079 and 82270099, with the funders remaining uninvolved in the research design and conduct.
Author information
Authors and Affiliations
Contributions
J. X. was responsible for the overall quality of all data in the research, ensuring both its integrity and the precision of its analysis. J. X. and Y. L. contributed to the scientific conception and design of the study. J. X., H. H. Z., H. H. L., Y. N. J., J. Z., M. W., and N. C. contributed to literature search; data collection, analysis, and interpretation; and the manuscript drafting. All authors reviewed and provided their final consent for the manuscript.
Corresponding authors
Ethics declarations
Ethical approval
All procedures involving human subjects adhered to the ethical standards set by the institutional and national research committees, as well as complied with the 1964 Helsinki Declaration and its later modifications, or analogous ethical standards.
Informed consent
All participants in the study provided informed consent.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, H., Liu, H., Jiao, Y. et al. Association between sleep apnea-specific hypoxic burden and severity of coronary artery disease. Sleep Breath (2024). https://doi.org/10.1007/s11325-024-03008-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11325-024-03008-1