Abstract
Purpose
Oral appliances (OA) are used to treat patients with obstructive sleep apnea (OSA). The purpose of this study is to evaluate the efficacy of OA treatment in patients with rapid eye movement (REM)–related OSA.
Methods
Forty-six patients with REM-related OSA and 107 with non-stage-specific OSA were prescribed OA treatment after diagnosis by polysomnography (PSG) and a follow-up sleep test by PSG was conducted. Efficacy and treatment outcome predictors were evaluated according to the following criteria for treatment success: #1, reduction of the apnea-hypopnea index (AHI) to less than 5 and > 50% compared with baseline; #2, AHI reduction to less than 10 and > 50% compared with baseline; and #3, > 50% AHI reduction compared with baseline.
Results
Success rates according to criteria #1, #2, and #3 were 45.7%, 50.0%, and 50.0% in REM-related OSA and 36.4%, 52.3%, and 63.6% in non-stage-specific OSA, respectively. No significant differences in success rate were found between the two groups. In multivariate logistic regression analysis with each criterion as the response variable, only BMI was extracted as a significant predictor. The BMI cutoff values defined based on the maximum Youden index according to the three criteria were 26.2 kg/m2, 25.6 kg/m2, and 26.2 kg/m2, respectively.
Conclusions
No significant differences in success rate of OA treatment were found between REM-related OSA and non-stage-specific OSA. BMI has greater impact on treatment outcome of OA in patients with REM-related OSA.
Similar content being viewed by others
Introduction
Obstructive sleep apnea (OSA), characterized by repetitive respiratory events including apnea and hypopnea, is due to total or partial collapse of the upper airways during sleep, and affects 9 to 38% of the general adult population [1]. Untreated OSA is associated with daytime symptoms, various comorbidities, and mortality [2]. Although continuous positive airway pressure (CPAP) is clearly a highly effective treatment option, various alternative treatment options are available, such as oral appliances, upper airway surgery, and hypoglossal nerve stimulation, with sufficient evidence supporting their use in selected patient populations [3]. Thus sleep medicine for OSA is moving into the era of personalized treatment.
Rapid eye movement (REM)–related OSA, a highly prevalent subtype of OSA affecting 13 to 36%of patients, is characterized by apnea and hypopnea events predominantly or exclusively occurring during REM sleep [4, 5]. Although the pathophysiology of REM-related OSA is still unclear and its overall severity as defined by the apnea and hypopnea index (AHI) tends to be mild to moderate, it should be evaluated separately from non-stage-specific OSA, because a recent cohort study indicated that REM-related OSA is independently associated with important cardiovascular risk factors such as hypertension, metabolic syndrome, and diabetes [6]. Therefore, appropriate management of these patients is crucial.
However, it is difficult to determine the appropriate course of management due to the lack of clinical data regarding the aforementioned treatment options for REM-related OSA. Moreover, two recent clinical studies have indicated that it is difficult for patients with REM-related OSA to achieve good adherence, and these patients are occasionally intolerant to continuous positive airway pressure (CPAP) therapy, which is the standard OSA treatment option [7, 8]. Hence, we consider it an urgent challenge to collect clinical data for each treatment option for this specific type of OSA.
The use of oral appliances (OA) in OSA treatment, and specifically of mandibular advancement devices, which prevent upper airway collapse by protruding the mandible forward and altering the tongue position, is supported by strong evidence [9]. Clinical guidelines recommend OA treatment for patients with mild to moderate OSA and for those with severe OSA who are intolerant to CPAP therapy or refuse it [10]. Therefore, sleep clinicians have many opportunities to prescribe OA for patients with REM-related OSA. However, only one study reported the efficacy of OA in REM-related OSA [11], so that the level of evidence about the efficacy of this treatment is low.
Cephalometric analysis is highly recommended in patients with OSA as one of the most important tools for diagnosis and treatment planning [12]. In addition, several studies reported that specific cephalometric measurements predict OA treatment outcome [13]. However, only one study evaluated the craniofacial characteristics of the patients with REM-related OSA using cephalometric measurements [14]. Moreover, no study evaluated cephalometric measurements as predictors of OA treatment success in patients with REM-related OSA.
The aim of this study was to evaluate the outcome of OA treatment and clarify its predictors in patients with REM-related OSA.
Materials and methods
Single-center retrospective observational study assessed patients who were prescribed OA after a diagnosis of OSA by polysomnography (PSG) at the Department of Sleep Medicine of the Aichi Medical University Hospital from January 2007 to December 2018. At the time of their first visit to the Department of Oral and Maxillofacial Surgery, all patients underwent craniofacial evaluation by cephalometry.
Nocturnal polysomnography
Diagnostic and follow-up nocturnal PSG was performed using the Alice 4 or 5 system (Respironics, Inc., Murrysville, PA, USA). The following biological variables were continuously monitored: electrocardiogram, electroencephalogram, chin and anterior tibialis electromyogram, bilateral electro-oculogram, airflow measurement using a nasal thermistor, arterial oxygen saturation, respiratory effort measured by thoracic and abdominal inductive plethysmography bands, body position, and snoring. Respiratory events, including apnea and hypopnea, and other PSG parameter, were scored manually by sleep technicians according to the 2007 guidelines of the American Academy of Sleep Medicine (AASM) [15]. The apnea-hypopnea index (AHI) was defined as the average number of apnea and hypopnea events per hour of sleep, and OSA was defined as AHI ≥ 5, following the International Classification of Sleep Disorder (ICSD)-2 criteria [16].
We defined REM-related OSA as an overall AHI ≥ 5, a ratio of AHI during REM sleep (AHIREM)/AHI during NREM sleep (AHINREM) ≥ 2, and AHINREM < 15, which is the definition most widely reported in the literature [4, 17,18,19].
Oral appliance
The OA was designed to protrude the mandible to maintain upper airway patency. A custom-made monobloc mandibular advancement oral appliance made from a 2.0-mm polyethylene plate (Erkodur; Erkodent Inc.; Pfalzgrafenweiler, Germany) was prescribed for all participants. The construction bite was registered at 70% of the maximum mandibular protruded position, while its vertical position was set at the minimum occlusal elevation that allowed for mandibular advancement [20].
Treatment outcome for OA was evaluated according to the following alternative criteria for success: #1, reduction of the AHI to a value < 5 and > 50% AHI reduction compared with baseline, the strictest criterion; #2, reduction of the AHI to a value < 10 and > 50% AHI reduction compared with baseline, the most frequently used criterion in the literature; and #3, > 50% AHI reduction compared with baseline [21, 22].
Cephalometric evaluation
Lateral cephalometric radiography was performed for all the participants in the upright position with the Frankfort horizontal plane parallel to the ground. The film was taken while holding their breath at the end of the inspiratory phase with closed lips and teeth in centric occlusion. A single investigator, blinded to the demographic and polysomnographic status of the participants, traced all cephalometric radiographs. The cephalometric landmarks and measurements used in this study are detailed in Fig. 1.
Cephalometric landmarks and reference planes: N (Nasion), S (sella), Ba (basion), Po (porion), Ar (articulare), Or (orbitale), Pt (pterygoid point), PNS (posterior nasal spine), A (point-A), B (point-B), Me (menton), Gn (gnathion), Go (gonion), and H (hyoid). FH, Frankfort horizontal plane; MP, mandibular plane; FP, facial plane. ① SNA (angle formed between sella, nasion, and point A), ② SNB (angle formed between sella, nasion, and point B), ③ facial axis (angle formed between FP and N-Ba), ④ mandibular plane angle (Angle formed between FH plane and mandibular plane), ⑤ gonion angle (angle formed between mandibular plane and Ar-Go). ⑥ S-N (linear distance between S and N), ⑦ Ar-Go (linear distance between Ar and Go), ⑧ Go-Me (linear distance between Go and Me), ⑨ MP-H (linear distance between the mandibular plane and H). ⑩ SPAS (width of the airway behind the soft palate along the line parallel to the Go-B line), ⑪ IAS (width of the airway along the Go-B line), ⑫ UD (soft palate thickness), and ⑬ UL (soft palate length)
Statistical analysis
All continuous variables were expressed as median (25th–75th percentile). The Student’s t test was used for normally distributed data, and the Mann-Whitney U test for non-normally distributed data. Normality was assessed using the Shapiro-Wilk test. Categorical variables were expressed as numbers (percentages) and compared using Fisher’s exact test. Predictive factors of OA treatment success were evaluated by multivariate logistic regression using the backward selection method, including all variables assessed in the study. The predictive ability was assessed by receiver operating characteristic (ROC) analysis using the area under curve (AUC). P values < 0.05 were considered statistically significant. Statistical analyses were performed using the SPSS software program for Windows, version 25.0 (SPSS Inc., Chicago, IL, USA) and the R statistical package (version 3.5.0, R Foundation for Statistical Computing).
Results
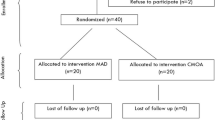
We assessed 1257 patients. Fifteen patients aged < 18 years, 561 patients who were followed up by out of center sleep testing (OCST), and 528 patients who did not undergo follow-up sleep testing were excluded. Ultimately, 153 adult OSA patients who underwent follow-up sleep testing by PSG were enrolled in the study (Fig. 2).
Out of 153 patients, 46 were REM-related OSA and 107 were non-stage-specific OSA. Table 1 shows the demographic and polysomnographic findings in patients with REM-related OSA and non-stage-specific OSA. Significant differences were found in sleep efficiency [87.1% (79.5–94.6) vs. 82.4% (70.8–91.7); p = 0.013], REM/total sleep time (TST) [18.7% (13.4–23.3) vs. 15.0% (11.9–15.0), p = 0.021], number of patients with 5 ≤ AHI < 15 [36 (78.3%) vs. 30 (28.0%), p < 0.001], number of patients with AHI ≥ 30 [0 (0%) vs. 36 (33.6%), p < 0.001], and percentage of time spent at SaO2 below 90% (CT90) [0.7% (0.1–0.7) vs. 1.3% (0.2–5.0), p = 0.039].
Table 2 shows the cephalometric findings in patients with REM-related OSA and non-stage-specific OSA. Significant differences were found in linear distance between articulare and gonion (Ar-Go) [54.9 mm (48.4–59.4) vs. 57.2 mm (52.5–61.3), p = 0.045] and between mandibular plane and hyoid distance (MP-H) [17.4 mm (11.8–19.8) vs. 18.2 mm (14.5–23.7), p = 0.036]. No significant differences were found in angular measurements.
Table 3 shows the success rate according to each criterion in REM-related OSA and non-stage-specific OSA. The success rates according to criteria #1, #2, and #3 were 45.7%, 50.0%, and 50.0% in REM-related OSA, and 36.4%, 52.3%, and 63.6% in non-stage-specific OSA, respectively. No significant differences in success rates were found between the two groups.
Table 4 shows the comparison of PSG findings, before and after OA treatment, in REM-related OSA and non-stage-specific OSA. The hypopnea index (HI) during REM sleep had no significant difference in both groups [REM-related OSA: 12.1 (3.7–23.1) vs. 5.2 (2.5–17.0), p = 0.183; non-stage-specific OSA: 6.7 (1.3–15.2) vs. 6.5 (1.4–17.9), p = 0.550].
Table 5 shows the results of the multivariate logistic regression analysis of the treatment outcome for REM-related OSA with the backward selection method including all variables assessed in this study. In the analysis with criterion #1 as the response variable, BMI [OR, 0.730; 95% CI, 0.559–0.955; p = 0.022] was independently associated with treatment success. In the analysis with criterion #2 as the response variable, BMI [OR, 0.732; 95% CI, 0.562–0.953; p = 0.02] was independently associated with treatment success. In the analysis with criterion #3 as the response variable, BMI [OR, 0.674; 95% CI, 0.501–0.907; p = 0.009] was independently associated with treatment success.
ROC curves were plotted to evaluate the predictive ability of BMI for each treatment success criterion in Fig 3. The areas under the ROC curve for BMI as a predictor of success according to criteria #1, #2, and #3 were 0.693 (95% CI, 0.540–0.847), 0.697 (0.542–0.852), and 0.724 (0.575-0.874), respectively. The best BMI cutoff values, defined as the maximum Youden index, according to criteria #1, #2, and #3 were 26.2 kg/m2 [sensitivity 95.2% (95% CI, 0.857–1.000), specificity 40.0%(95% CI, 0.240–0.600)], 25.6 kg/m2 [sensitivity 87.5% (95% CI, 0.750–1.000), specificity 45.5% (95% CI, 0.227–0.636)], and 26.2 kg/m2 [sensitivity 95.7% (95% CI, 0.870–1.000), specificity 43.5% (95% CI, 0.217–0.652)], respectively.
Discussion
To our knowledge, this is the first study evaluating predictors of treatment outcome for OA in patients with REM-related OSA, and also the first study evaluating OA treatment outcome and craniofacial characteristics in Japanese patients with REM-related OSA.
In craniofacial evaluation, REM-related OSA had significantly superior hyoid position compared with non-stage-specific OSA as measured by the distance from mandibular plane and hyoid bone (MP-H). An inferiorly displaced hyoid as measured by MP-H has been consistently associated with OSA severity [23]. Therefore, this result might reflect lower and stable AHI during NREM sleep which is major pathophysiological characteristics of REM-related OSA. In addition, none of the angular measurements, including sella–nasion–A point angle (SNA), sella–nasion–B point angle (SNB), facial axis, mandibular plane angle, and gonion angle, showed significant differences between the two groups. Only one previous study by Eun et al. evaluated craniofacial differences using 5 cephalometric measurements between the two groups, and also found no significant differences in any of the angular measurements assessed [14]. Although it is still unclear whether REM-related OSA has craniofacial characteristics different from non-stage-specific OSA, due to the limited data available, these results might indicate important aspects of the craniofacial characteristics of REM-related OSA. Further studies will be necessary to confirm our results, and will also contribute to elucidating the unknown pathophysiology of REM-related OSA.
Only the previous study by Sutherland et al. evaluated OA treatment outcomes for patients with REM-related OSA, and showed that complete response, defined by a reduction of the AHI to less than 5 events/h, which generally can be achieved by CPAP, was only observed in 12% of the patients [11]. On the other hand, this study showed that complete response, defined by the most stringent criterion #1, was observed in 45.7% of the patients. From these results, we conclude that OA could be a treatment option for selected patients with REM-related OSA.
Several studies have reported the association between higher BMI and poor response to OA treatment [24, 25]. Therefore, we hypothesize that the difference in success rates between the study by Sutherland et al. and our own were mainly due to the higher BMI (30.0 ± 5.3 kg/m2) of the participants in the former study compared with our sample, as shown in Table 1. Sutherland et al. also reported that REM-related OSA showed a lower success rate than non-stage specific OSA [11]. In this study, no significant differences in success rate were found between the two groups for any criterion. In addition, the data in Table 4 indicates significant differences in the change of AHI, AI, maximum desaturation from baseline, and cumulative percentage of time spent at saturation below 90% (CT90) during REM and NREM sleep, before and after OA treatment, in both group. These data suggest similar efficacy of OA for REM-related OSA and non-stage-specific OSA in our non-obese sample.
Patients with REM-related OSA who did not respond to the OA treatment were analyzed in more detail. These patients had difficulty reducing their AHI during REM sleep, which decreased only from 36.2/h (24.4–44.2) to 23.9/h (16.7–37.2). Considering separately the apnea index (AI) and the hypopnea index (HI), we observed that the AI during REM sleep was sufficiently reduced from 26.3/h (6.1–33.0) to 7.1/h (1.4–14.9), but the HI was actually increased from 8.6/h (2.4–22.1) to 12.4/h (4.4–28.9). These results indicated that OA can prevent complete upper airway collapse, but partial collapse may retain during REM sleep.
Clinically, it is well known that obesity, by increasing upper airway vulnerability due to soft tissue crowdedness, leads to poor response to OA treatment [24]. Consistently with previous reports, BMI was a significant predictor of treatment outcome for OA when using 3 different criteria for treatment success as the response variable. Moreover, more than 10 cephalometric measurements have been reported as predictors of treatment outcome, but no cephalometric measurements were found to be significant predictors of treatment outcome in this study [13]. These results suggest that soft tissue crowdedness has more impact than craniofacial abnormalities on the effect of mandibular advancement in patients with REM-related OSA. ROC analysis showed that roughly the same cutoff values, as defined by the maximum Youden index, were obtained in this study for the three success criteria. These cutoff values could be used to select the patients for whom OA could be an effective treatment option for REM-related OSA.
Our study has several limitations. First, the cephalometry conducted in this study was performed under wakefulness and offers only a two-dimensional image in the upright position. This examination can sufficiently analyze the craniofacial bony enclosure, but does not reveal the characteristics of soft tissue inside the craniofacial bony enclosure during sleep. We plan to perform a similar study including craniofacial variables determined by magnetic imaging and computed tomography under sedation. A second limitation was the selection bias due to this study being conducted in a single facility. Third, the effects of unknown confounding factors, including the use of medication affecting REM sleep or decreasing muscle tone, could not be excluded because of the retrospective nature of this study. Further studies are needed to confirm the external validity of our results.
Conclusion
No significant differences in success rate of OA treatment were founded between REM-related OSA and non-stage-specific OSA. BMI has more impact than craniofacial abnormality on treatment outcome in the patient with REM-related OSA. Cutoff values for BMI examined in this study could be used to select the patients for whom OA could be an effective treatment option. Further studies will be necessary to confirm the general applicability of our results.
References
Senaratna CV, Perret JL, Lodge CJ, Lowe AJ, Campbell BE, Matheson MC, Hamilton GS, Dharmage SC (2017) Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev 34:70–81. https://doi.org/10.1016/j.smrv.2016.07.002
Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, D’Agostino RB, Newman AB, Lebowitz MD, Pickering TG (2000) Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community based study: Sleep Heart Health Study. JAMA 283:1829–1836
Randerath W, Bassetti CL, Bonsignore MR, Farre R, Ferini-Strambi L, Grote L, Hedner J, Kohler M, Martinez-Garcia MA, Mihaicuta S, Montserrat J, Pepin JL, Pevernagie D, Pizza F, Polo O, Riha R, Ryan S, Verbraecken J, McNicholas WT (2018) Challenges and perspectives in obstructive sleep apnoea: Report by an ad hoc working group of the Sleep Disordered Breathing Group of the European Respiratory Society and the European Sleep Research Society. Eur Respir J 52:1702616. https://doi.org/10.1183/13993003.02616-2017
Conwell W, Patel B, Doeing D, Pamidi S, Knutson KL, Ghods F, Mokhlesi B (2012) Prevalence, clinical features, and CPAP adherence in REM-related sleep-disordered breathing: a cross-sectional analysis of a large clinical population. Sleep Breath 16:519–526. https://doi.org/10.1007/s11325-011-0537-6
Mano M, Hoshino T, Sasanabe R, Murotani K, Nomura A, Hori R, Konishi N, Baku M, Shiomi T (2019) Impact of gender and age on rapid eye movement-related obstructive sleep apnea: a clinical study of 3234 Japanese OSA patients. Int J Environ Res Public Health 16:E1068. https://doi.org/10.3390/ijerph16061068
Acosta-Castro P, Hirotsu C, Marti-Soler H, Marques-Vidal P, Tobback N, Andries D, Waeber G, Preisig M, Vollenweider P, Haba-Rubio J, Heinzer R (2018) REM-associated sleep apnoea: prevalence and clinical significance in the HypnoLaus cohort. Eur Respir J 52:1702484. https://doi.org/10.1183/13993003.02484-2017
Almeneessier AS, Almousa Y, Hammad O, Olaish AH, ALAnbay ET, BaHammam AS (2017) Long-term adherence to continuous positive airway pressure in patients with rapid eye movement-only obstructive sleep apnea: a prospective cohort study. J Thorac Dis 9:3755–3765. https://doi.org/10.21037/jtd.2017.09.57
Hoshino T, Sasanabe R, Tanigawa T, Murotani K, Arimoto M, Ueda H, Shiomi T (2018) Effect of rapid eye movement related obstructive sleep apnea on adherence to continuous positive airway pressure. J Int Med Res 46:2238–2248. https://doi.org/10.1177/0300060518758583
Sutherland K, Vanderveken OM, Tsuda H, Marklund M, Gagnadoux F, Kushida CA, Cistulli PA (2014) Oral appliance treatment for obstructive sleep apnea: an update. J Clin Sleep Med 10:215–227. https://doi.org/10.5664/jcsm.3460
Ramar K, Dort LC, Katz SG, Lettieri CJ, Harrod CG, Thomas SM, Chervin RD (2015) Clinical practice guideline for the treatment of obstructive sleep apnea and snoring with oral appliance therapy: an update for 2015. J Clin Sleep Med 11:773–827. https://doi.org/10.5664/jcsm.4858
Sutherland K, Takaya H, Qian J, Petocz P, Ng AT, Cistulli PA (2015) Oral appliance treatment response and polysomnographic phenotypes of obstructive sleep apnea. J Clin Sleep Med 11:861–868. https://doi.org/10.5664/jcsm.4934
Tangugsorn V, Skatvedt O, Krogstad O, Lyberg T (1995) Obstructive sleep apnea: a cephalometric study. PartII. Uvulo-glossopharyngeal morphology. Eur J Orthod 17:57–67
Alessandri-Bonetti G, Ippolito DR, Bartolucci ML, D’Antò V, Incerti-Parenti S (2015) Cephalometric predictors of obstructive sleep apnea: a systematic review. Korean J Orthod 45:308–321. https://doi.org/10.4041/kjod.2015.45.6.308
Eun YG, Kwon KH, Shin SY, Lee KH, Byun JY, Kim SW (2009) Multilevel surgery in patients with rapid eye movement-related obstructive sleep apnea. Otolaryngol Head Neck Surg 140:536–541. https://doi.org/10.1016/j.otohns.2009.01.006
Iber C, Ancoli-Israel S, Chesson AL Jr, Quan SF (2007) The AASM Manual for the Scoring of Sleep and Associated Events. American Academy of Sleep Medicine, Westchester
American Academy of Sleep Medicine (2005) International Classification of Sleep Disorders. In: diagnostic and coding manual, 2nd edn. American Academy of Sleep, Westchester, pp 79–94
Sakao S, Sakurai T, Yahaba M, Sakurai Y, Terada J, Tanabe N, Tatsumi K (2015) Features of REM-related sleep disordered breathing in the Japanese population. Intern Med 54:1481–1487. https://doi.org/10.2169/internalmedicine.54.4248
Hoshino T, Sasanabe R, Mano M, Nomura A, Kato C, Sato M, Imai M, Murotani K, Guilleminault C, Shiomi T (2019) Prevalence of rapid eye movement-related obstructive sleep apnea in adult narcolepsy. Intern Med 58:2151–2157. https://doi.org/10.2169/internalmedicine.2601-18
Lee SA, Paek JH, Han SH (2016) REM-related sleep-disordered breathing is associated with depressive symptoms in men but not in women. Sleep Breath 20:995–1002. https://doi.org/10.1007/s11325-016-1323-2
Furuhashi A, Yamada S, Shiomi T, Sasanabe R, Aoki Y, Yamada Y, Kazaoka Y (2013) Effective three-dimensional evaluation analysis of upper airway form during oral appliance therapy in patients with obstructive sleep apnoea. J Oral Rehabil 40:582–589. https://doi.org/10.1111/joor.12059
Ferguson KA, Cartwright R, Rogers R, Schmidt-Nowara W (2006) Oral appliances for snoring and obstructive sleep apnea: a review. Sleep 29:244–262
Fukuda T, Tsuiki S, Kobayashi M, Nakayama H, Inoue Y (2014) Selection of response criteria affects the success rate of oral appliance treatment for obstructive sleep apnea. Sleep Med 15:367–370. https://doi.org/10.1016/j.sleep.2013.12.007
Genta PR, Schorr F, Eckert DJ et al (2014) Upper airway collapsibility is associated with obesity and hyoid position. Sleep 37:1673–1678
Tsuiki S, Ito E, Isono S, Ryan CF, Komada Y, Matsuura M, Inoue Y (2013) Oropharyngeal crowding and obesity as predictors of oral appliance treatment response to moderate obstructive sleep apnea. Chest 144:558–563. https://doi.org/10.1378/chest.12-2609
Liu Y, Lowe AA, Fleetham JA, Park YC (2001) Cephalometric and physiologic predictors of the efficacy of an adjustable oral appliance for treating obstructive sleep apnea. Am J Orthod Dentofac Orthop 120:639–647
Acknowledgments
The authors wish to thank the patients who participated in this study, the dental technicians who fabricate OA, including Atsushi Isaji and Yuji Morishita, and the sleep technicians who manually scored the PSG data, including Yoko Murakami, Jyunko Hiraki, Takehiro Yamaguchi, Aki Arita, Chihiro Kato, Msako Sato, and Masato Imai.
Author information
Authors and Affiliations
Contributions
Yoshitomo Nishio designed the study, and wrote the initial draft of the manuscript. Tetsuro Hoshino contributed to analysis and interpretation of date, and assisted in the preparation of the manuscript. All other authors have contributed to interpretation, data collection, and critically reviewed the manuscript. All authors approved the final version of the manuscript, and agree to be accountable for all aspect of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional review board (IRB) of Aichi Medical University Hospital (approval number 19-H006) and adhered to the tenets of the 1964 Declaration of Helsinki.
Informed consent
The IRB granted a waiver of informed consent due to the non-invasive and retrospective nature of the study. Nevertheless, we published an outline of the study plan which is available for public viewing on the Aichi Medical University Web site in order to provide patients with the opportunity to decline participation; however, none of the patients declined to participate in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Nishio, Y., Hoshino, T., Murotani, K. et al. Treatment outcome of oral appliance in patients with REM-related obstructive sleep apnea. Sleep Breath 24, 1339–1347 (2020). https://doi.org/10.1007/s11325-019-01966-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-019-01966-5