Abstract
Purpose
Insomnia is an important adverse event of mechanical thromboprophylaxis. This sleep disorder has been reported as one of the commonest adverse events of the new oral anti-Xa anticoagulant darexaban, with similar rates to mechanical thromboprophylaxis in a randomized controlled trial (RCT). However, the perceived effect could have been biased because it was an open-label RCT. Therefore, we aimed to review the incidence of insomnia with non-vitamin K antagonist oral anticoagulants (NOACs).
Methods
We performed a systematic review and meta-analysis of Phase III RCTs. Electronic databases MEDLINE and CENTRAL (inception to September 2013) were searched as well as review articles and references of included studies.
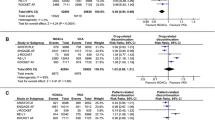
We included phase III RCTs which compared NOACs with any other control group. Data were analyzed and pooled to estimate risk ratio (RR) with 95% confidence intervals (95%CI) for insomnia using inverse variance method. Statistical heterogeneity was evaluated with I 2 test.
Results
We included seven studies (two apixaban RCTs, two dabigatran RCTs, one darexaban RCTs, and two rivaroxaban RCTs), enrolling a total of 23,023 patients. Overall, NOACs were not associated to an increased risk of insomnia: RR 0.94 (95%CI 0.83–1.08; I 2 = 0%). In blinded studies (six studies), NOACs also did not show increased risk of insomnia (RR 0.94, 95%CI 0.83–1.08; I 2 = 0%). Results were similar irrespective of the comparators.
Conclusions
NOACs (apixaban, dabigatran, darexaban, rivaroxaban) did not show increased risk of insomnia. Results according to study design (blinded vs. open-label trials) overlap the main analysis.




Similar content being viewed by others
References
Ancoli-Israel S, Roth T (1999) Characteristics of insomnia in the United States: results of the 1991 National Sleep Foundation survey. I Sleep 22(Suppl 2):S347–53
Broström A, Strömberg A, Dahlström U, Fridlund B (2004) Sleep difficulties, daytime sleepiness, and health-related quality of life in patients with chronic heart failure. J Cardiovasc Nurs 19:234–42
Byles JE, Mishra GD, Harris MA, Nair K (2003) The problems of sleep for older women: changes in health outcomes. Age Ageing 32:154–63
Sakon M, Nakamura M (2012) Darexaban (YM150) prevents venous thromboembolism in Japanese patients undergoing major abdominal surgery: phase III randomized, mechanical prophylaxis-controlled, open-label study. Thromb Res 130:e52–9
Cindolo L, Salzano L, Mirone V, Imbimbo C, Longo N, Kakkos SK, Reddy DJ (2009) Thromboprophylaxis in radical retropubic prostatectomy: efficacy and patient compliance of a dual modality. Urol Int 83:12–8
Pildal J, Hróbjartsson A, Jørgensen KJ, Hilden J, Altman DG, Gøtzsche PC (2007) Impact of allocation concealment on conclusions drawn from meta-analyses of randomized trials. Int J Epidemiol 36:847–57
Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, Gluud C, Martin RM, Wood AJ, Sterne JA (2008) Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ 336:601–5
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339:b2700
Higgins JPT, Altman DG, Sterne JAC (2011) Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration. http://www.cochrane-handbook.org. Acessed in December 2013
Deeks JJ (2002) Issues in the selection of a summary statistic for meta-analysis of clinical trials with binary outcomes. Stat Med 21:1575–600
Deeks JJ, Altman DG, Bradburn MJ (2001) Statistical methods for examining heterogeneity and combining results from several studies in metaanalysis. In Egger M, Davey Smith G, Altman DG, editors. Systematic reviews in health care: meta-analysis in context, 2nd ed. London: BMJ Publication Group; p. 313–335
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–34
Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L (2006) Comparison of two methods to detect publication bias in meta-analysis. JAMA 295:676–80
Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Portman RJ (2009) Apixaban or enoxaparin for thromboprophylaxis after knee replacement. N Engl J Med 361:594–604
Alexander JH, Lopes RD, James S, Kilaru R, He Y, Mohan P, Bhatt DL, Goodman S et al (2011) Apixaban with antiplatelet therapy after acute coronary syndrome. N Engl J Med 365:699–708
Eriksson BI, Dahl OE, Rosencher N, Kurth AA, van Dijk CN, Frostick SP, Prins MH, Hettiarachchi R, Hantel S, Schnee J, Büller HR; RE-NOVATE Study Group (2007) Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet. 15;370:949–56
Eriksson BI, Dahl OE, Huo MH, Kurth AA, Hantel S, Hermansson K, Schnee JM, Friedman RJ, Study Group RE-NOVATEII (2011) Oral dabigatran versus enoxaparin for thromboprophylaxis after primary total hip arthroplasty (RE-NOVATE II*). A randomised, double-blind, non-inferiority trial. Thromb Haemost 105:721–9
Eriksson BI, Borris LC, Friedman RJ, Haas S, Huisman MV, Kakkar AK, Bandel TJ, Beckmann H, Muehlhofer E, Misselwitz F, Geerts W; RECORD1 Study Group (2008) Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med. 26;358:2765–75.
Lassen MR, Ageno W, Borris LC, Lieberman JR, Rosencher N, Bandel TJ, Misselwitz F, Turpie AG, RECORD3 Investigators (2008) Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med 358:2776–86
Sterne JAC, Egger M, Moher D (2011) Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration. http://www.cochrane-handbook.org. Acessed in December 2013
McAinsh J, Cruickshank JM (1990) Beta-blockers and central nervous system side effects. Pharmacol Ther 46:163–97
Chang CH, Yang YH, Lin SJ, Su JJ, Cheng CL, Lin LJ (2013) Risk of insomnia attributable to β-blockers in elderly patients with newly diagnosed hypertension. Drug Metab Pharmacokinet 28:53–8
Funding
This was an academic project not funded by government or non-government grants.
Author contributions
DC contributed to the concept and design, data acquisition, data analysis, and interpretation of the data; wrote the first draft of the manuscript; critically revised the manuscript; and gave final approval of the submitted manuscript. MB, DdA, and ATS contributed to the data acquisition, data analysis; critically revised the manuscript; and gave final approval of the submitted manuscript. JC and JJF contributed to the concept and design, interpretation of the data, critically revised the manuscript, and gave final approval of the submitted manuscript.
Conflict of interest
DC, MB, DdA, ATS, and JC do not have any competing interests to disclose. JJF had speaker and consultant fees with GlaxoSmithKline, Novartis, Lundbeck, Solvay, Abbott, Bial, Grunenthal, and Merck Sharp and Dohme.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 719 kb)
Supplemental Figure 1
Risk of bias of included studies. (TIFF 191 kb)
Supplemental Figure 2
Funnel plot with included studies. (TIFF 114 kb)
Rights and permissions
About this article
Cite this article
Caldeira, D., Barra, M., Santos, A.T. et al. Risk of insomnia with non-vitamin K oral anticoagulants: systematic review and meta-analysis. Sleep Breath 19, 1043–1049 (2015). https://doi.org/10.1007/s11325-014-1112-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-014-1112-8