Abstract
Purpose
The degree and dynamic progression of neuroinflammation after traumatic spinal cord injuries (SCI) are crucial determinants of the severity of injury and potential for recovery. We used Positron Emission Tomography (PET) to monitor neuroinflammation longitudinally, correlating it with Chemical Exchange Saturation Transfer (CEST) Magnetic Resonance Imaging (MRI) and behavior in contusion-injured rats. These studies help validate CEST metrics and confirm how imaging may be used to evaluate the efficacy of therapies and understand their mechanisms of action.
Procedures
12 SCI and 4 sham surgery rats were subjected to CEST MRI and PET-Translocator Protein (TSPO) scans for 8 weeks following injury. Z-spectra from the SCI were analyzed using a 5-Lorentzian pool model for fitting. Weekly motor and somatosensory behavior were correlated with imaging metrics, which were validated through post-mortem histological and immuo-staining using ionized calcium-binding adaptor protein-1 (iba-1, microglia) and glial fibrillary acidic protein (GFAP, astrocytes).
Results
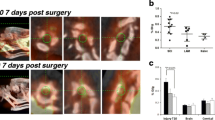
PET-TSPO showed widespread inflammation and post-mortem histology confirmed the presence of activated microglia. Changes in CEST and nuclear Overhauser Effect (NOE) peaks at 3.5 ppm and -1.6 ppm respectively were largest within the first week after injury and more pronounced in rostral versus caudal segments. These temporal indices of neuroinflammation corresponded to the recovery of locomotor behaviors and somatic sensation in rats with moderate contusion injury. The results confirm that CEST MRI metrics are sensitive indices of states of neuroinflammation within injured spinal cords.
Conclusions
The detection of dynamic spatiotemporal features of neuroinflammation progression underscores the importance of considering their timings and locations for neuroprotective and anti-inflammatory therapies. The availability of noninvasive MRI indices of neuroinflammation may facilitate clinical trials aimed at treatments that promote recovery after SCI.





Similar content being viewed by others
Data availability
The datasets acquired from the study and analysis code used are available from the corresponding author upon reasonable request.
References
Silva NA, Sousa N, Reis RL, Salgado AJ (2014) From basics to clinical: A comprehensive review on spinal cord injury. Prog Neurobiol 114:25–57. https://doi.org/10.1016/j.pneurobio.2013.11.002
Sweis R, Biller J (2017) Systemic Complications of Spinal Cord Injury. Curr Neurol Neurosci Rep 17:1–8
Profyris C, Cheema SS, Zang DW et al (2004) Degenerative and regenerative mechanisms governing spinal cord injury. Neurobiol Dis 15:415–436. https://doi.org/10.1016/J.NBD.2003.11.015
Badhiwala JH, Ahuja CS, Fehlings MG (2019) Time is spine: A review of translational advances in spinal cord injury. J Neurosurg Spine 30:1–18
Stammers AT, Liu J, Kwon BK (2012) Expression of inflammatory cytokines following acute spinal cord injury in a rodent model. J Neurosci Res 90:782–790. https://doi.org/10.1002/JNR.22820
Cohen-Adad J, El Mendili M-M, Lehéricy S et al (2011) Demyelination and degeneration in the injured human spinal cord detected with diffusion and magnetization transfer MRI. Neuroimage 55:1024–1033. https://doi.org/10.1016/J.NEUROIMAGE.2010.11.089
Fawcett JW, Asher RA (1999) The glial scar and central nervous system repair. Brain Res Bull 49:377–391
Yiu G, He Z (2006) Glial inhibition of CNS axon regeneration. Nat Rev Neurosci 7:617–627
van Zijl PCM, Yadav NN (2011) Chemical exchange saturation transfer (CEST): what is in a name and what isn’t? Magnetic Resonance in Medicine 65(4):927–948. https://doi.org/10.1002/mrm.22761
Wang F, Qi HX, Zu Z et al (2015) Multiparametric MRI reveals dynamic changes in molecular signatures of injured spinal cord in monkeys. Magn Reson Med 74:1125–1137. https://doi.org/10.1002/mrm.25488
Wang F, Zu Z, Wu R et al (2018) MRI evaluation of regional and longitudinal changes in Z-spectra of injured spinal cord of monkeys. Magn Reson Med 79:1070–1082. https://doi.org/10.1002/mrm.26756
Wu TL, Byun NE, Wang F et al (2020) Longitudinal assessment of recovery after spinal cord injury with behavioral measures and diffusion, quantitative magnetization transfer and functional magnetic resonance imaging. NMR Biomed 33:e4216. https://doi.org/10.1002/NBM.4216
Zhou J, Heo HY, Knutsson L et al (2019) APT-weighted MRI: Techniques, current neuro applications, and challenging issues. J Magn Reson Imaging 50:347–364. https://doi.org/10.1002/JMRI.26645
Zhang XY, Wang F, Afzal A et al (2016) A new NOE-mediated MT signal at around -1.6 ppm for detecting ischemic stroke in rat brain. Magn Reson Imaging 34:1100–1106. https://doi.org/10.1016/j.mri.2016.05.002
Tremoleda JL, Thau-Zuchman O, Davies M et al (2016) In vivo PET imaging of the neuroinflammatory response in rat spinal cord injury using the TSPO tracer [18F]GE-180 and effect of docosahexaenoic acid. Eur J Nucl Med Mol Imaging 43:1710–1722. https://doi.org/10.1007/s00259-016-3391-8
Narayanaswami V, Dahl K, Bernard-Gauthier V et al (2018) Emerging PET radiotracers and targets for imaging of neuroinflammation in neurodegenerative diseases: Outlook beyond TSPO. Molecular Imaging 2018;17. https://doi.org/10.1177/1536012118792317
Werry EL, Bright FM, Piguet O et al (2019) Recent Developments in TSPO PET Imaging as A Biomarker of Neuroinflammation in Neurodegenerative Disorders. Int J Mol Sci 20:3161. https://doi.org/10.3390/IJMS20133161
Lu M, Drake G, Wang F et al (2021) Design and construction of an interchangeable RF coil system for rodent spinal cord MR imaging at 9.4 T. Magn Reson Imaging 84:124–131. https://doi.org/10.1016/j.mri.2021.09.015
O’Haver T (2008) peakfit.m
Cheung YY, Nickels ML, Tang D et al (2014) Facile synthesis of SSR180575 and discovery of 7-chloro-N, N,5-trimethyl-4-oxo-3(6-[18F]fluoropyridin-2-yl)-3,5-dihydro-4H-pyridazino[4,5-b]indole-1-acetamide, a potent pyridazinoindole ligand for PET imaging of TSPO in cancer. Bioorg Med Chem Lett 24:4466–4471. https://doi.org/10.1016/J.BMCL.2014.07.091
Fouad K, Ng C, Basso DM (2020) Behavioral testing in animal models of spinal cord injury. Exp Neurol 333:113410
Deuis JR, Dvorakova LS, Vetter I (2017) Methods Used to Evaluate Pain Behaviors in Rodents. Front Mol Neurosci 10:284. https://doi.org/10.3389/fnmol.2017.00284
Bankhead P, Loughrey MB, Fernández JA et al (2017) (2017) QuPath: Open source software for digital pathology image analysis. Sci Rep 71(7):1–7. https://doi.org/10.1038/s41598-017-17204-5
Liu D, Thangnipon W, McAdoo DJ (1991) Excitatory amino acids rise to toxic levels upon impact injury to the rat spinal cord. Brain Res 547:344–348. https://doi.org/10.1016/0006-8993(91)90984-4
Iizuka H, Yamamoto H, Iwasaki Y, Konno H (1987) Evolution of tissue damage in compressive spinal cord injury in rats. J Neurosurg 66:595–603. https://doi.org/10.3171/JNS.1987.66.4.0595
Tietze A, Blicher J, Mikkelsen IK et al (2014) Assessment of ischemic penumbra in patients with hyperacute stroke using amide proton transfer (APT) chemical exchange saturation transfer (CEST) MRI. NMR Biomed 27:163–174. https://doi.org/10.1002/nbm.3048
Jones CK, Schlosser MJ, van Zijl PCM et al (2006) Amide proton transfer imaging of human brain tumors at 3T. Magn Reson Med 56:585–592. https://doi.org/10.1002/mrm.20989
Zhang H, Wang W, Jiang S et al (2017) Amide proton transfer-weighted MRI detection of traumatic brain injury in rats. J Cereb Blood Flow Metab 37:3422–3432. https://doi.org/10.1177/0271678X17690165/ASSET/IMAGES/LARGE/10.1177_0271678X17690165-FIG1.JPEG
Dong Y, Gu Y, Lu J et al (2022) Amide Proton Transfer-Weighted Magnetic Resonance Imaging for Detecting Severity and Predicting Outcome after Traumatic Brain Injury in Rats. Neurotrauma Reports 3:261–275. https://doi.org/10.1089/NEUR.2021.0064/ASSET/IMAGES/LARGE/NEUR.2021.0064_FIGURE7.JPEG
Dickens AM, Vainio S, Marjamäki P et al (2014) Detection of Microglial Activation in an Acute Model of Neuroinflammation Using PET and Radiotracers 11C-(R)-PK11195 and 18F-GE-180. J Nucl Med 55:466–472. https://doi.org/10.2967/JNUMED.113.125625
Lavisse S, Guillermier M, Hérard AS et al (2012) Reactive Astrocytes Overexpress TSPO and Are Detected by TSPO Positron Emission Tomography Imaging. J Neurosci 32:10809–10818. https://doi.org/10.1523/JNEUROSCI.1487-12.2012
Toossi A, Bergin B, Marefatallah M et al (2021) (2021) Comparative neuroanatomy of the lumbosacral spinal cord of the rat, cat, pig, monkey, and human. Sci Reports 111(11):1–15. https://doi.org/10.1038/s41598-021-81371-9
Kim J, Wu Y, Guo Y et al (2015) A review of optimization and quantification techniques for chemical exchange saturation transfer MRI toward sensitive in vivo imaging. Contrast Med Mol Imaging 10:163–178. https://doi.org/10.1002/CMMI.1628
Duan W, Huang Q, Chen Z et al (2019) Comparisons of motor and sensory abnormalities after lumbar and thoracic contusion spinal cord injury in male rats. Neurosci Lett 708:134358. https://doi.org/10.1016/J.NEULET.2019.134358
Gaudet AD, Ayala MT, Schleicher WE et al (2017) Exploring acute-to-chronic neuropathic pain in rats after contusion spinal cord injury. Exp Neurol 295:46–54. https://doi.org/10.1016/J.EXPNEUROL.2017.05.011
Cai K, Haris M, Singh A et al (2012) Magnetic resonance imaging of glutamate. Nat Med 182(18):302–306. https://doi.org/10.1038/nm.2615
By S, Barry RL, Smith AK et al (2018) Amide proton transfer CEST of the cervical spinal cord in multiple sclerosis patients at 3T. Magn Reson Med 79:806–814. https://doi.org/10.1002/MRM.26736
Acknowledgements
This study is supported by DOD Grant SC190134 (awarded to LMC) and acknowledges the support also of NIH award 1 S10 OD025085 (awarded to JCG). We acknowledge additional NIH support for instrumentation 1S10OD016245-01 and 1 S10RR023784-01. The authors gratefully acknowledge Zou Yue and Chaohui Tang for their assistance with surgical procedures and perfusion of animals, Drs. Yiu-Yin Cheung and Fei Liu and Radiochemistry core for radiotracer production, the Center for Small Animal Imaging (CSAI) staff for imaging support, and the Vanderbilt University Medical Center (VUMC) Translational Pathology Shared Resource Core (NCI/NIH Cancer Center Support Grant 2P30 CA068485-14) for their expertise with processing the spinal cord tissue histology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All applicable institutional and/or national guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The manuscript has been submitted for review and approved for submission by all of the named co-authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mu, C., Reed, J.L., Wang, F. et al. Spatiotemporal Dynamics of Neuroinflammation Relate to Behavioral Recovery in Rats with Spinal Cord Injury. Mol Imaging Biol 26, 240–252 (2024). https://doi.org/10.1007/s11307-023-01875-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-023-01875-w