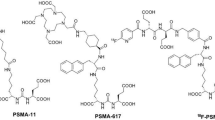
Abstract
Purpose
Investigate the impact of various halogens on pharmacokinetics, biodistribution, and micro positron emission tomography/computed tomography (PET/CT) imaging of Glu-urea-Lys-based prostate-specific membrane antigen (PSMA) inhibitors.
Procedures
Based on the modification of SC691, a small molecule inhibitor of PSMA previously developed by our group, we synthesized 68Ga-labeled compounds by modifying the lysine terminal amino with different halogenated phenyl substituents. After complete characterization, in vitro and in vivo properties were studied.
Results
The [68Ga]Ga-DOTA-SC691-R possesses a high radiochemical yield (98–99%). The internalization values of [68Ga]Ga-DOTA-SC691-H, [68Ga]Ga-DOTA-SC691-Cl, and [68Ga]Ga-DOTA-SC691-Br in LNCaP cells all displayed time-dependent pattern enhanced with time. The results of in vitro competitive inhibition assay showed that the affinity of natGa-DOTA-SC691-R for PSMA had a trend of H < F < Cl < Br < I. The blocking imaging and dynamic imaging on micro-PET/CT of male non-obese diabetic/severe combined immunodeficiency mice with LNCaP tumors showed the rapid tumor targeting properties of [68Ga]Ga-DOTA-SC691-R with specificity for PSMA. Static imaging of micro-PEC/CT of these compounds could rapidly localize LNCaP tumors with decent image quality (except for [68Ga]Ga-DOTA-SC691-H). Biodistribution data showed that [68Ga]Ga-DOTA-SC691-R were metabolized via the kidney and tumor accumulation followed the order of H ≈ F ≈ Cl < I < Br uptake values at 1 h. [68Ga]Ga-DOTA-SC691-Br showed the highest tumor accumulation and retention (15.21 ± 5.57%ID/g at 30 min, 20.39 ± 4.38%ID/g at 60 min, and 13.30 ± 4.39%ID/g at 120 min), which is consistent with the results of the competitive inhibition assay and cell binding assay.
Conclusions
It was demonstrated that the halogen substituent on the lysine terminal amino group on the Glu-urea-Lys backbone did positively affect the binding of [68Ga]Ga-DOTA-SC691-R to PSMA. The bulkier and less electronegative Br (or I) elements are preferred for structural modifications here.






Similar content being viewed by others
References
Siegel RL, Miller KD, Fuchs HE, Jemal A (2022) Cancer statistics, 2022. CA Cancer J Clin. 72(1):7–33
Sung H, Ferlay J, Siegel RL et al (2021) Global Cancer Statistics 2020: GLOBOCAN Estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 71(3):209–249
Siegel RL, Miller KD, Fuchs HE, Jemal A (2021) Cancer statistics, 2021. CA Cancer J Clin. 71(1):7–33
Auchus RJ, Sharifi N (2020) Sex hormones and prostate cancer. Annu Rev Med. 71:33–45
Chatalic KL, Konijnenberg M, Nonnekens J et al (2016) In vivo stabilization of a gastrin-releasing peptide receptor antagonist enhances PET imaging and radionuclide therapy of prostate cancer in preclinical studies. Theranostics. 6(1):104–117
Tsechelidis I, Vrachimis A (2022) PSMA PET in imaging prostate cancer. Front Oncol. 12:831429
Barve A, Jin W, Cheng K (2014) Prostate cancer relevant antigens and enzymes for targeted drug delivery. J Control Release. 187:118–132
Kiess AP, Banerjee SR, Mease RC et al (2015) Prostate-specific membrane antigen as a target for cancer imaging and therapy. Q J Nucl Med Mol Imaging. 59(3):241–268
Perera M, Papa N, Roberts M et al (2020) Gallium-68 prostate-specific membrane antigen positron emission tomography in advanced prostate cancer-updated diagnostic utility, sensitivity, specificity, and distribution of prostate-specific membrane antigen-avid lesions: a systematic review and meta-analysis. Eur Urol. 77(4):403–417
Maurer T, Eiber M, Schwaiger M, Gschwend JE (2016) Current use of PSMA-PET in prostate cancer management. Nat Rev Urol. 13(4):226–235
Jeitner TM, Babich JW, Kelly JM (2022) Advances in PSMA theranostics. Transl Oncol. 22:101450
Chen H, Cai P, Feng Y et al (2021) In vitro and in vivo comparative study of a novel 68Ga-labeled PSMA-targeted inhibitor and 68Ga-PSMA-11. Sci Rep. 11:19122
Chang SS, Reuter VE, Heston WD et al (1999) Five different anti-prostate-specific membrane antigen (PSMA) antibodies confirm PSMA expression in tumor-associated neovasculature. Cancer Res. 59(13):3192–3198
Chang SS (2004) Overview of prostate-specific membrane antigen. Rev Urol. 6(Suppl 10):S13–S18
Zhou J, Neale JH, Pomper MG, Kozikowski AP (2005) NAAG peptidase inhibitors and their potential for diagnosis and therapy. Nat Rev Drug Discov. 4(12):1015–1026
Okarvi SM (2019) Recent developments of prostate-specific membrane antigen (PSMA)-specific radiopharmaceuticals for precise imaging and therapy of prostate cancer: an overview. Clin Transl Imaging. 7(3):189–208
Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C (1997) Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 3(1):81–85
Bostwick DG, Pacelli A, Blute M, Roche P, Murphy GP (1998) Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma: a study of 184 cases. Cancer. 82(11):2256–2261
Eder M, Eisenhut M, Babich J, Haberkorn U (2013) PSMA as a target for radiolabelled small molecules. Eur J Nucl Med Mol Imaging. 40(6):819–823
Mesters JR, Barinka C, Li W et al (2006) Structure of glutamate carboxypeptidase II, a drug target in neuronal damage and prostate cancer. EMBO J. 25(6):1375–1384
Barinka C, Hlouchova K, Rovenska M et al (2008) Structural basis of interactions between human glutamate carboxypeptidase II and its substrate analogs. J of Mol Bio 376(5):1438–1450
Barinka C, Starkova J, Konvalinka J, Lubkowski J (2007) A high-resolution structure of ligand-free human glutamate carboxypeptidase II. Acta Crystallographica Section F-Struc Bio Com 63:150–153
Barinka C, Byun Y, Dusich CL et al (2008) Interactions between human glutamate carboxypeptidase II and urea-based inhibitors: structural characterization. J Med Chem. 51(24):7737–7743
Neels OC, Kopka K, Liolios C, Afshar-Oromieh A (2021) Radiolabeled PSMA inhibitors. Cancers (Basel). 13(24)
Pastorino S, Riondato M, Uccelli L et al (2020) Toward the discovery and development of PSMA targeted inhibitors for nuclear medicine applications. Curr Radiopharm. 13(1):63–79
Chen Y, Foss CA, Byun Y et al (2008) Radiohalogenated prostate-specific membrane antigen (PSMA)-based ureas as imaging agents for prostate cancer. J Med Chem. 51(24):7933–7943
Maresca KP, Hillier SM, Femia FJ et al (2009) A series of halogenated heterodimeric inhibitors of prostate specific membrane antigen (PSMA) as radiolabeled probes for targeting prostate cancer. J Med Chem. 52(2):347–357
Banerjee SR, Kumar V, Lisok A et al (2019) 177Lu-labeled low-molecular-weight agents for PSMA-targeted radiopharmaceutical therapy. Eur J Nucl Med Mol Imaging. 46(12):2545–2557
Kwon H, Lim H, Ha H et al (2020) Structure-activity relationship studies of prostate-specific membrane antigen (PSMA) inhibitors derived from alpha-amino acid with (S)- or (R)-configuration at P1′ region. Bioorg Chem. 104:104304
Plechanovova A, Byun Y, Alquicer G et al (2011) (2011) Novel substrate-based inhibitors of human glutamate carboxypeptidase II with enhanced lipophilicity. J Med Chem. 54(21):7535–7546
Funding
The authors would like to thank the National Natural Science Foundation of China for funding this work (U20A20384), the Sichuan Science and Technology Foundation (2021YJ0131), the Doctoral Research Initiation Fund of Affiliated Hospital of Southwest Medical University, Nuclear Medicine and Molecular Imaging Key Laboratory of Sichuan Province (HYX19001), and the Luzhou-Southwest Medical University Cooperative Application Foundation (2020LZXNYDJ50).
Author information
Authors and Affiliations
Contributions
Conceptualization: Zhijun Zhou and Yue Chen; methodology: Li Xia, Nan Liu, and Zhijun Zhou; validation: Li Xia and Yang Liu; formal analysis: Zhijun Zhou and Li Xia; investigation: Li Xia, Yang Liu, Ping Cai, Yue Feng, Hongmei Yuan, Sufan Tang, and Yinwen Wang; data curation: Li Xia and Yang Liu; writing: Li Xia; funding acquisition: Yue Chen and Zhijun Zhou; resources: Yue Chen and Zhijun Zhou; review: Zhijun Zhou, Li Xia, Yang Liu, Ping Cai, Yue Feng, Hongmei Yuan, Sufan Tang, Yinwen Wang, Yue Chen, and Nan Liu.
Corresponding authors
Ethics declarations
Ethics Approval
The Ethics Committee for Southwest Medical University (2021-02-29) approved the study. All methods were carried out following relevant guidelines and regulations, and all methods are reported per ARRIVE guidelines.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1:
Suppl. Fig. 1 1H NMR of DOTA-SC691-H. Suppl. Fig. 2 Mass Spectroscopy of DOTA-SC691-H. Suppl. Fig. 3 1H NMR of DOTA-SC691-F. Suppl. Fig. 4 Mass Spectroscopy of DOTA-SC691-F. Suppl. Fig. 5 1H NMR of DOTA-SC691-Cl. Suppl. Fig. 6 Mass Spectroscopy of DOTA-SC691-Cl. Suppl. Fig. 7 1H NMR of DOTA-SC691-Br. Suppl. Fig. 8 Mass Spectroscopy of DOTA-SC691-Br. Suppl. Fig. 9 1H NMR of DOTA-SC691-3FM. Suppl. Fig. 10 Mass Spectroscopy of DOTA-SC691-3FM. Suppl. Fig. 11 1H NMR of DOTA-SC691-6KL. Suppl. Fig. 12 Mass Spectroscopy of DOTA-SC691-6KL. Suppl. Fig. 13 1H NMR of DOTA-SC691-7KL. Suppl. Fig. 14 Mass Spectroscopy of DOTA-SC691-7KL. Suppl. Fig. 15 Stability of [68Ga]Ga-DOTA-SC691-R. Suppl. Fig. 16 Displacement curves of natGa-DOTA-SC691-R and natGa-PSMA-11 competitive inhibition of [68Ga]Ga-PSMA-11 binding to LNCaP cells. Suppl. Fig. 17 %ID/gmean curves of dynamic Micro-PET/CT scans. Suppl. Fig. 18 Micro-PET/CT images of blocking studies. Suppl. Table 1 Organ biodistribution of [68Ga]Ga-DOTA-SC691-Br and [68Ga]Ga-DOTA-SC691-I. (DOCX 21376 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xia, L., Liu, Y., Cai, P. et al. Halogen Replacement on the Lysine Side Chain of Lys-Urea-Glu-Based PSMA Inhibitors Leads to Significant Changes in Targeting Properties. Mol Imaging Biol 25, 765–775 (2023). https://doi.org/10.1007/s11307-023-01804-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-023-01804-x