Abstract
Introduction
Citrus canker, a disease caused by Xanthomonas axonopodis pv. citri (Xac) bacteria, has been responsible for extensive economic losses in citriculture. In this work, we report the metabolic responses of citrus plants during disease development. This information can be useful for understanding the natural mechanism of plant defense beyond helping design new varieties and/or genetically modified genotypes for tolerance/resistance against citrus canker.
Objectives
To understand how primary metabolism is affected in two sweet orange genotypes during citrus canker development.
Methods
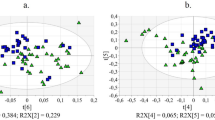
1H NMR spectroscopy together with chemometrics was used to evaluate the metabolic changes caused by Xac infection at various time points (days 4, 12 and 20) in Citrus sinensis L. Osbeck leaves from non-transgenic and transgenic plants expressing the antibacterial peptide sarcotoxin.
Results
The results revealed a high level of metabolic similarity between the studied genotypes without Xac infection. However, after Xac infection, the plants responded differently to disease development. The non-transgenic genotype showed altered early precursors of some secondary metabolites (tryptophan, tyrosine and putrescine) in addition to signaling metabolites of biotic stress (putrescine and dimethylamine), and the drastic reduction of gluconeogenesis was the overall metabolic cost for defense. The transgenic genotype suffered late metabolic changes due to the protective stoichiometric role of sarcotoxin. In addition, the oxidative stress response was more balanced in transgenic than in non-transgenic plants.
Conclusion
An NMR-based metabolomic approach was useful for understanding plant–pathogen interactions in citrus canker. Our findings provide valuable preliminary insights into different stages of citrus canker development.





Similar content being viewed by others
References
Allwood, J. W., Ellis, D. I., & Goodacre, R. (2008). Metabolomic technologies and their application to the study of plants and plant-host interactions. Physiologia Plantarum, 132, 117–135.
Bespalhok Filho, J. C., Kobayashi, A. K., Pereira, L. F. P., & Vieira, L. G. E. (2001). Laranja transgênica: Transformação de laranja visando resistência ao cancro cítrico usando genes de peptídeos antibacterianos. Biotecnologia Ciência & Desenvolvimento, 28, 229–234.
Boscariol, R. L., Monteiro, M., Takahashi, E. K., Chabregas, S. M., Vieira, M. L. C., Vieira, L. G. E., Mourão-Filho, F. A. A., Cardoso, S. C., Christiano, R. S. C., Bergamin Filho, A., Barbosa, J. M., Azevedo, F. A., & Mendes, B. M. J. (2006). Attacin A gene from Tricloplusia ni reduces susceptibility to Xanthomonas axonopodis pv. citri in transgenic Citrus sinensis ‘Hamlin’. Journal of the American Society for Horticultural Science, 131(4), 530–536.
Brunnings, A. M., & Gabriel, D. W. (2003). Xanthomonas citri: Breaking the surface. Molecular Plant Pathology, 3(4), 141–157.
Cernadas, R. A., Camillo, L. R., & Benedetti, C. E. (2008). Transcriptional analysis of the sweet orange interaction with the citrus canker pathogens Xanthomonas axonopodis pv. citri and Xanthomonas axonopodis pv. aurantifolii. Molecular Plant Pathology, 9(5), 609–631.
Cevallos-Cevallos, J. M., Futch, D. B., Shilts, T., Folimonova, J. I., & Reyes-De-Corcuera, J. I. (2012). GC-MS metabolomic differentiation of selected citrus varieties with diferente sensitivity to citrus huanglongbing. Plant Physiology and Biochemistry, 53, 69–76.
Choi, Y. H., Tapias, E. C., Hye, K. K., Lefeber, A. W. M., Erkelens, C., Verhoeven, J. T. J., et al. (2004). Metabolic discrimination of Catharanthus roseus leaves infected by phytoplasma using 1H-NMR spectroscopy and multivariate data analysis. Plant Physiology, 135, 2398–2410.
D’Mello, J. P. F. (2015). Amino acids in higher plants. Edinburgh: Cabi Publishing.
Dutta, T. K., Papolu, P. K., Banakar, P., Choudhary, D., Sirohi, A., & Rao, U. (2015). Tomato transgenic plants expressing hairpin construct of a nematode protease gene conferred enhanced resistance to root-knot nematodes. Frontiers in Microbiology, 6(260), 1–14.
Fabro, G., Kovács, I., Pavet, V., Szabados, L., & Alvarez, M. E. (2004). Proline accumulation and AtP5CS2 gene activation are induced by plant-pathogen incompatible interactions in Arabidopsis. American Phytopathological Society, 17(4), 343–350.
Genoud, T., & Métraux, J. -P. (1999). Crosstalk in plant cell signaling: Structure and function of the genetic network. Trends in Plant Science, 4(12), 503–507.
Gonsalves, D., Gonsalves, C., Ferreira, S., Pitz, K., Fitch, M., Manshardt, R., & Slightom, J. (2004). Transgenic virus resistant papaya: From hope to reality for controlling papaya ringspot virus in Hawaii. American Phytopathological Society. Retrieved August 21, 2016 from http://oregonstate.edu/instruct/bi430-fs430/Documents-2004/3B-BIOTECH%20METH/Gonsalves-papaya-story-AmPhytopSoc2004.pdf.
Gottwald, T. R., Graham, J. H., & Schubert, T. S. (2002). Citrus canker: The pathogen and its impact. Plant Health Progress. Retrieved August 20, 2016 from https://www.plantmanagementnetwork.org/pub/php/review/citruscanker/.
Grosser, J. W., Dutt, M., Omar, A., Orbovic, V., & Barthe, G. (2011). Progress towards the development of transgenic disease resistance in citrus. Acta Horticulturae, 892, 101–107.
Grover, R., & Gowthaman, R. (2003). Strategies for development of fungus-resistant transgenic plants. Current Science, 84(3), 330–340.
Hare, P. D., & Cress, W. A. (1997). Metabolic implications of stress-induced proline accumulation in plants. Plant Growth Regulation, 21, 79–102.
Hwang, I. S., An, S. H., & Hwang, B. K. (2011). Pepper asparagine synthetase 1 (CaAS1) is required for plant nitrogen assimilation and defense responses to microbial pathogens. The Plant Journal, 67, 749–762.
Itai, C., & Paleg, L. G. (1982). Responses of water-stressed Hordeum distichum L. and Cucumis sativus to proline and betaine. Plant Science Letters, 25, 329–335.
Khalaf, A., Moore, G. A., Jones, J. B., & Gmitter, F. G. J. (2007). New insights into the resistance of Nagami kumquat to canker disease. Physiological and Molecular Plant Pathology, 71, 240–0250.
Kumar, N., Ebel, R. C., & Roberts, P. D. (2011). H2O2 metabolism during sweet orange (Citrus sinensis L. Osb.) ‘Hamlin’ Xanthomonas axonopodis pv. citri interaction. Scientia Horticulturae, 128, 465–472.
Lima, M. R. M., Felgueiras, M. L., Graca, G., Rodrigues, J. E. A., Barros, A., Gil, A. M., & Dias, A. C. P. (2010). NMR metabolomics of esca disease-affected Vitis vinifera cv. Alvarinho leaves. Journal of Experimental Botany, 61, 4033–4042.
Nicholson, J. K., Holmes, E., & Lindon, J. C. (2007). Chapter 1: Metabonomics and metabolomics techniques and their applications in mammalian systems. In the handbook of metabonomics and metabolomics (pp. 1–34). Elsevier.
Ohshima, M., Mitsuhara, I., Okamoto, M., Sawano, S., Nishiyama, K., Kaku, H., et al. (1999). Enhanced resistance to bacterial diseases of transgenic tobacco plants overexpressing sarcotoxin IA, a bactericidal peptide of insect. The Journal of Biochemistry, 125, 431–435.
Poulson-Ellestad, K. L., Jones, C. M., Roy, J., Viant, M. R., Fernández, F. M., Kubanek, J., & Nunn, B. L. (2014). Metabolomics and proteomics reveal impacts of chemically mediated competition on marine plankton. Proceedings of the National Academy of Sciences of the United States of America, 111(24), 9009–9014.
Rhodes, D., & Hanson, A. D. (1993). Quaternary ammonium and tertiary sulfonium compounds in higher plants. Annual Reviews of Plant Physiology and Plant Molecular, 44, 357–384.
Rojas, C. M., Senthil-Kumar, M., Tzin, V., & Mysore, K. S. (2014). Regulation of primary plant metabolism during plant-pathogen interactions and its contribution to plant defense. Frontiers in Plant Science, 5, 1–12.
Rossi, F. R., Mariana, M., & Pieckenstain, F. L. (2015). Role of arginine decarboxylase (ADC) in Arabidopsis thaliana defence against the pathogenic bacterium Pseudomonas viridiflava. Plant Biology, 17, 831–839.
Silva, L. M. A., Alves, E. G. F., Choze, R., Lião, L. M., & Alcantara, G. B. (2012). 1H HRMAS NMR spectroscopy and chemometrics for evaluation of metabolic changes in Citrus sinensis caused by Xanthomonas axonopodis pv. citri. Journal of the Brazilian Chemical Society, 23(6), 1054–1061.
Skopelitis, D. S., Paranychianakis, N. V., Paschalidis, K. A., Pliakonis, E. D., Delis, I. D., Yakoumakis, D. I., et al. (2006). Abiotic stress generates ROS that signal expression of anionic glutamate dehydrogenases to form glutamate for proline synthesis in tobacco and grapevine. The Plant Cell Online, 18(10), 2767–2781.
Summers, P. S., & Weretilnyk, E. A. (1993). Choline synthesis in relation to salt stress. Plant Physiology, 103, 1269–1276.
Szabados, L., & Savouré, A. (2009). Proline: A multifunctional amino acid. Cell Press, 15, 89–97.
Turrens, J. F. (2003). Mitochondrial formation of reactive oxygen species. Journal of Physiology, 552(2), 335–344.
Uawisetwathana, U., Graham, S. F., Kamolsukyunyong, W., Sukhaket, W., Klanchui, A., Toojinda, T., et al. (2015). Quantitative 1H NMR metabolome profiling of Thai Jasmine rice (Oryza sativa) reveals primary metabolic response during brown planthopper infestation. Metabolomics. doi:10.1007/s11306-015-0817-4.
Wally, O., & Punja, Z. K. (2010). Genetic engineering for increasing fungal and bacterial disease resistance in crop plants. Genetically Modified Crops, 1(4), 199–206.
Wink, M. (2010). Biochemistry of plant secondary metabolism. Chichester: Wiley.
Winter, G., Todd, C. D., Trovato, M., Foriani, G., & Funck, D. (2015). Physiological implications of arginine metabolism in plant. Frontiers in Plant Science, 6, 1–14.
Zhang, J., Zhang, Y., Du, Y., Chen, S., & Tang, H. (2011). Dynamic metabonomic responses of tobacco (Nicotiana tabacum) plants to salt stress. Journal of Proteome Research, 10(4), 1904–1914.
Acknowledgements
This work received financial support from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Apoio ao Desenvolvimento do Ensino, Ciência e Tecnologia do Estado de Mato Grosso do Sul (FUNDECT).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no competing interests.
Ethical approval
This article does not contain any studies with human or animal subjects.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
do Prado Apparecido, R., Carlos, E.F., Lião, L.M. et al. NMR-based metabolomics of transgenic and non-transgenic sweet orange reveals different responses in primary metabolism during citrus canker development. Metabolomics 13, 20 (2017). https://doi.org/10.1007/s11306-017-1163-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11306-017-1163-5