Abstract
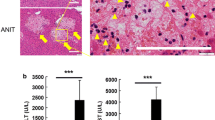
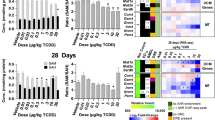
Understanding mechanisms of liver injury can enable better preclinical testing and clinical management of patients. Carbon tetrachloride (CCl4), used extensively as a model hepatotoxicant, induces lipid perturbation and increases in plasma bile acids (BAs). An integrated transcriptomics and metabolomics approach was employed to investigate CCl4-induced alterations in lipid and BA metabolism. Sprague–Dawley rats were treated orally with corn oil, 50 (low dose, LD) or 2,000 mg CCl4/kg/d (high dose, HD). Animals were sacrificed at 6, 24 or 72 h. Terminal blood was collected for clinical chemistry and metabolomics analyses. Livers were harvested for histopathology, metabolomics and transcriptomics analyses. Both alanine aminotransferase (ALT) and aspartate aminotransferase (AST) increased in the treated groups with the greatest increases observed in the HD group at 24 and 72 h. Blood cholesterol and triglycerides (TGs) were significantly decreased in the HD group at both 24 and 72 h, and hepatocyte vacuolization was observed at these timepoints. Consistent with the clinical chemistry and histopathological data, metabolomics results showed that levels of total fatty acids increased in the liver but decreased in the blood in the HD group at the 24 and 72 h timepoints. This suggested that lipids accumulate in the liver. Primary BAs increased in both liver and blood, while secondary and conjugated BAs decreased in the liver and increased in the blood, which indicated that the BA conjugation pathway and that BA uptake by the liver were inhibited by CCl4. Results from this study provide a better understanding of the mechanisms of CCl4-induced hepatotoxicity.



Similar content being viewed by others
References
Bai, C. L., & Stacey, N. H. (1993). Effects of carbon tetrachloride and chloroform on bile acid transport in isolated rat hepatocytes: Relationship to elevated serum bile acids. Toxicology in Vitro, 7, 197–203.
Basu, S. (2003). Carbon tetrachloride-induced lipid peroxidation: Eicosanoid formation and their regulation by antioxidant nutrients. Toxicology, 189, 113–127.
Bauer, A., Schumann, A., Gilbert, M., Wilhelm, C., Hengstler, J. G., et al. (2009). Evaluation of carbon tetrachloride-induced stress on rat hepatocytes by 31P NMR and MALDI-TOF mass spectrometry: Lysophosphatidylcholine generation from unsaturated phosphatidylcholines. Chemistry and Physics of Lipids, 159, 21–29.
Bechtold, M. M., Gee, D. L., Bruenner, U., & Tappel, A. L. (1982). Carbon tetrachloride-mediated expiration of pentane and chloroform by the intact rat: The effects of pretreatment with diethyl maleate, SKF-525A and phenobarbital. Toxicology Letters, 11, 165–171.
Boll, M., Weber, L. W., Becker, E., & Stampfl, A. (2001). Hepatocyte damage induced by carbon tetrachloride: Inhibited lipoprotein secretion and changed lipoprotein composition. Zeitschrift fur Naturforschung C, 56, 283–290.
Chung, H., Hong, D. P., Kim, H. J., Jang, K. S., Shin, D. M., et al. (2005). Differential gene expression profiles in the steatosis/fibrosis model of rat liver by chronic administration of carbon tetrachloride. Toxicology and Applied Pharmacology, 208, 242–254.
Eastmond, D. A. (2008). Evaluating genotoxicity data to identify a mode of action and its application in estimating cancer risk at low doses: A case study involving carbon tetrachloride. Environmental and Molecular Mutagenesis, 49, 132–141.
Huang, X., Shao, L., Gong, Y., Mao, Y., Liu, C., et al. (2008). A metabonomic characterization of CCl4-induced acute liver failure using partial least square regression based on the GC/MS metabolic profiles of plasma in mice. Journal of Chromatography B Analytical Technologies in the Biomedical and Life Sciences, 870, 178–185.
Lee, P. Y., McCay, P. B., & Hornbrook, K. R. (1982). Evidence for carbon tetrachloride-induced lipid peroxidation in mouse liver. Biochemical Pharmacology, 31, 405–409.
Letteron, P., Labbe, G., Degott, C., Berson, A., Fromenty, B., et al. (1990). Mechanism for the protective effects of silymarin against carbon tetrachloride-induced lipid peroxidation and hepatotoxicity in mice. Evidence that silymarin acts both as an inhibitor of metabolic activation and as a chain-breaking antioxidant. Biochemical Pharmacology, 39, 2027–2034.
Lin, Y., Si, D., Zhang, Z., & Liu, C. (2009). An integrated metabonomic method for profiling of metabolic changes in carbon tetrachloride induced rat urine. Toxicology, 256, 191–200.
Manibusan, M. K., Odin, M., & Eastmond, D. A. (2007). Postulated carbon tetrachloride mode of action: A review. Journal of Environmental Science and Health—Part C Environmental Carcinogenesis & Ecotoxicology Reviews, 25, 185–209.
Martinez, M., Mourelle, M., & Muriel, P. (1995). Protective effect of colchicine on acute liver damage induced by CCl4. Role of cytochrome P-450. Journal of Applied Toxicology, 15, 49–52.
Mourelle, M., Villalon, C., & Amezcua, J. L. (1988). Protective effect of colchicine on acute liver damage induced by carbon tetrachloride. Journal of Hepatology, 6, 337–342.
Pan, X., Hussain, F. N., Iqbal, J., Feuerman, M. H., & Hussain, M. M. (2007). Inhibiting proteasomal degradation of microsomal triglyceride transfer protein prevents CCl4-induced steatosis. Journal of Biological Chemistry, 282, 17078–17089.
Pan, L., Qiu, Y., Chen, T., Lin, J., Chi, Y., et al. (2010). An optimized procedure for metabonomic analysis of rat liver tissue using gas chromatography/time-of-flight mass spectrometry. Journal of Pharmaceutical and Biomedical Analysis, 52, 589–596.
Plaa, G. L. (2000). Chlorinated methanes and liver injury: Highlights of the past 50 years. Annual Review of Pharmacology and Toxicology, 40, 42–65.
Pound, A. W., & Lawson, T. A. (1975). Reduction of carbon tetrachloride toxicity by prior administration of a single small dose in mice and rats. British Journal of Experimental Pathology, 56, 172–179.
Rao, K. S., & Recknagel, R. O. (1969). Early incorporation of carbon-labeled carbon tetrachloride into rat liver particulate lipids and proteins. Experimental and Molecular Pathology, 10, 219–228.
Rechnagel, R. O., & Glende, E. A, Jr. (1973). Carbon tetrachloride hepatotoxicity: An example of lethal cleavage. CRC Critical Reviews in Toxicology, 2, 263–297.
Recknagel, R. O. (1967). Carbon tetrachloride hepatotoxicity. Pharmacological Reviews, 19, 145–208.
Sun, J., Schnackenberg, L. K., Hansen, D. K., & Beger, R. D. (2010). Study of valproic acid-induced endogenous and exogenous metabolite alterations using LC-MS-based metabolomics. Bioanalysis, 2, 207–216.
Sun, J., Von Tungeln, L. S., Hines, W., & Beger, R. D. (2009). Identification of metabolite profiles of the catechol-O-methyl transferase inhibitor tolcapone in rat urine using LC/MS-based metabonomics analysis. Journal of Chromatography B-Analytical Technologies in the Biomedical and Life Sciences, 877, 2557–2565.
Tribble, D. L., Aw, T. Y., & Jones, D. P. (1987). The pathophysiological significance of lipid peroxidation in oxidative cell injury. Hepatology, 7, 377–386.
Vulimiri, S. V., Berger, A., & Sonawane, B. (2011). The potential of metabolomic approaches for investigating mode(s) of action of xenobiotics: Case study with carbon tetrachloride. Mutation Research, 722, 147–153.
Weber, L. W., Boll, M., & Stampfl, A. (2003). Hepatotoxicity and mechanism of action of haloalkanes: Carbon tetrachloride as a toxicological model. Critical Reviews in Toxicology, 33, 105–136.
Weddle, C. C., Hornbrook, K. R., & McCay, P. B. (1976). Lipid peroxidation and alteration of membrane lipids in isolated hepatocytes exposed to carbon tetrachloride. Journal of Biological Chemistry, 251, 4973–4978.
Wong, F. W., Chan, W. Y., & Lee, S. S. (1998). Resistance to carbon tetrachloride-induced hepatotoxicity in mice which lack CYP2E1 expression. Toxicology and Applied Pharmacology, 153, 109–118.
Acknowledgments
The authors thank Alan Warbritton for expert technical assistance with the photomicrographs.
Conflict of interests
All authors (J. Sun, T. Schmitt, L. K. Schnackenberg, L. Pence, Y. Ando, J. Greenhaw, X. Yang, S. Slavov, K. J. Davis, W. F. Salminen, D. L. Mendrick, and R. D. Beger) declare that they have no conflict of interest.
Disclaimer
The views presented in this article do not necessarily reflect those of the U.S. Food and Drug Administration.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sun, J., Schmitt, T., Schnackenberg, L.K. et al. Comprehensive analysis of alterations in lipid and bile acid metabolism by carbon tetrachloride using integrated transcriptomics and metabolomics. Metabolomics 10, 1293–1304 (2014). https://doi.org/10.1007/s11306-014-0665-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11306-014-0665-7