Abstract
Purpose
To evaluate arterial spin labeling (ASL) and diffusion-weighted imaging (DWI) in discrimination of benign from malignant paranasal sinus (PNS) tumors.
Material and methods
A prospective study was done upon 42 cases of PNS masses that underwent magnetic resonance ASL and DWI of the head. Tumor blood flow (TBF) and apparent diffusion coefficient (ADC) of the masses were calculated by two observers. The pathological diagnosis was malignant (n = 28) and benign (n = 14) cases.
Results
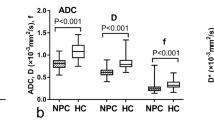
For both observers, the malignant PNS masses had significantly higher TBF (P < 0.001, 0.001) and lower ADC (P < 0.001, 0.001) than in benign masses. The ROC curve analysis of TBF, The threshed TBF was (121.45, 122.68 mL/100 g/min) used for differentiation between benign and malignant PNS masses, revealed sensitivity (92.9%, 89.3%), specificity (85.7%, 85.7%), accuracy (90.5%, 88.1%) and the AUC was 0.87 and 0.86 by both observers. the ROC curve analysis of ADC, The threshold ADC (1.215, 1.205 X10−3mm2/s) was used for differentiation between benign and malignant PNS masses, revealed sensitivity (96.4%, 89.3%), specificity (78.6%, 78.6%), accuracy of (90.5%, 85.7%) and the AUC was 0.93 and 0.92 by both observers. Combined analysis of TBF and ADC used for differentiation between benign and malignant PNS masses had revealed sensitivity (96.4%, 89.3%), specificity (92.9%, 85.7%) accuracy of (95.2%, 88.1%) and AUC.
(0.995, 0.985) for both observers.
Conclusion
Combined using of TBF and ADC have a role in differentiation malignant from benign PNS masses.




Similar content being viewed by others
References
Oysu AS, Aygün N. Imaging of Nasal Cavity and Paranasal Sinus Tumors. In: Cemal Cingi, Nuray Bayar Muluk (EDS) All Around the Nose. Cham: Springer; 2020. p. 149–67.
Agarwal M, Policeni B. Sinonasal Neoplasms. Semin Roentgenol. 2019;54:244–57.
Sasaki M, Eida S, Sumi M, Nakamura T. Apparent diffusion coefficient mapping for sinonasal diseases: differentiation of benign and malignant lesions. AJNR Am J Neuroradiol. 2011;32:1100–6.
Sanghvi S, Khan MN, Patel NR, Yeldandi S, Baredes S, Eloy JA. Epidemiology of sinonasal squamous cell carcinoma: a comprehensive analysis of 4994 patients. Laryngoscope. 2014;124:76–83.
Jegoux F, Metreau A, Louvel G, Bedfert C. Paranasal sinus cancer. Eur Ann Otorhinolaryngol Head Neck Dis. 2014;130:327–35.
Pal I, Gupta A, Sengupta S. An attempt to define the type of biopsy in a sinonasal lesion showing bony erosion. Indian J Otolaryngol Head Neck Surg. 2010;62:92–5.
Razek AA, Tawfik A, Elsorogy L, Soliman N. Perfusion CT of head and neck cancer. Eur J Radiol. 2014;83:537–44.
Abdel Razek AA, Gaballa G, Ashamalla G, Alashry MS, Nada N. Dynamic susceptibility contrast perfusion-weighted magnetic resonance imaging and diffusion-weighted magnetic resonance imaging in differentiating recurrent head and neck cancer from postradiation changes. J Comput Assist Tomogr. 2015;39:849–54.
Razek AA, Nada N. Correlation of choline/creatine and apparent diffusion coefficient values with the prognostic parameters of head and neck squamous cell carcinoma. NMR Biomed. 2016;29:483–9.
Abdel Razek AA, Poptani H. MR spectroscopy of head and neck cancer. Eur J Radiol. 2013;82:982–9.
Telischak NA, Detre JA, Zaharchuk G. Arterial spin labeling MRI: clinical applications in the brain. J Magn Reson Imaging. 2015;41:1165–80.
Abdel Razek AAK, Talaat M, El-Serougy L, Gaballa G, Abdelsalam M. Clinical applications of arterial spin labeling in brain tumors. J Comput Assist Tomogr. 2019;43(525):32.
Fujima N, Kudo K, Tsukahara A, Yoshida D, Sakashita T, Homma A, et al. Measurement of tumor blood flow in head and neck squamous cell carcinoma by pseudo-continuous arterial spin labeling: Comparison with dynamic contrast-enhanced MRI. J Magn Reson Imaging. 2015;41:983–91.
Abdel Razek AAK, Nada N. Arterial spin labeling perfusion-weighted MR imaging: correlation of tumor blood flow with pathological degree of tumor differentiation, clinical stage and nodal metastasis of head and neck squamous cell carcinoma. Eur Arch Otorhinolaryngol. 2018;275:1301–7.
Abdel Razek AAK. Arterial spin labelling and diffusion-weighted magnetic resonance imaging in differentiation of recurrent head and neck cancer from post-radiation changes. J Laryngol Otol. 2018;132:923–8.
Fujima N, Yoshida D, Sakashita T, Homma A, Tsukahara A, Tha KK, et al. Usefulness of Pseudocontinuous arterial spin-labeling for the assessment of patients with head and neck squamous cell carcinoma by measuring tumor blood flow in the pretreatment and early treatment period. AJNR Am J Neuroradiol. 2016;37:342–8.
Razek AA. Assessment of masses of the external ear with diffusion weighted MR imaging. Otol Neurotol. 2018;39:227–31.
Abdel Razek AA, Kamal E. Nasopharyngeal carcinoma: correlation of apparent diffusion coefficient value with prognostic parameters. Radiol Med. 2013;118:534–9.
Abdel Razek A, Mossad A, Ghonim M. Role of diffusion-weighted MR imaging in assessing malignant versus benign skull-base lesions. Radiol Med. 2011;116:125–32.
Abdel Razek A, Elkhamary S, Al-Mesfer S, AlKatan H. Correlation of apparent diffusion coefficient at 3 tesla with prognostic parameters of retinoblastoma. Am J Neuroradiol. 2012;33:944–8.
Razek AA, El-Serougy L, Abdelsalam M, Gaballa G, Talaat M. Differentiation of residual/recurrent gliomas from postradiation necrosis with arterial spin labeling and diffusion tensor magnetic resonance imaging-derived metrics. Neuroradiology. 2018;60:169–77.
Fujima N, Nakamaru Y, Sakashita T, Homma A, Tsukahara A, Kudo K, et al. Differentiation of squamous cell carcinoma and inverted papilloma using non-invasive MR perfusion imaging. Dentomaxillofac Radiol. 2015;44:20150074.
Fujima N, Kameda H, Tsukahara A, Yoshida D, Sakashita T, Homma A, et al. Diagnostic value of tumor blood flow and its histogram analysis obtained with pCASL to differentiate sinonasal malignant lymphoma from squamous cell carcinoma. Eur J Radiol. 2015;84:2187–93.
Razek AAKA, Sieza S, Maha B. Assessment of nasal and paranasal sinus masses by diffusion-weighted MR imaging. J Neuroradiol. 2009;36:206–11.
Das A, Bhalla AS, Sharma R, Kumar A, Thakar A, Vishnubhatla SM, et al. Can diffusion weighted imaging aid in differentiating benign from malignant sinonasal masses?: a useful adjunct. Polish J Radiol. 2017;82:345–55.
Razek KA, AA. Characterization of salivary gland tumours with diffusion tensor imaging. Dentomaxillofac Radiol. 2018;47:20170343.
Abdel Razek AAK. Routine and advanced diffusion imaging modules of the salivary glands. Neuroimaging Clin N Am. 2018;28:245–54.
Abdel Razek AAK, El-Serougy L, Abdelsalam M, Gaballa G, Talaat M. Differentiation of primary central nervous system lymphoma from glioblastoma: quantitative analysis using arterial spin labeling and diffusion tensor imaging. World Neurosurg. 2019;123:e303-9.
Razek AAKA, Talaat M, El-Serougy L, Abdelsalam M, Gaballa G. Differentiating glioblastomas from solitary brain metastases using arterial spin labeling perfusion− and diffusion tensor imaging− derived metrics. World Neurosurg. 2019;127:593–8.
Razek AAKA. Multi-parametric MR imaging using pseudo-continuous arterial-spin labeling and diffusion-weighted MR imaging in differentiating subtypes of parotid tumors. Magn Reson Imaging. 2019;63:55–9.
Razek AAKA, Helmy E. Multi-parametric arterial spin labeling and diffusion-weighted imaging in differentiation of metastatic from reactive lymph nodes in head and neck squamous cell carcinoma. Eur Arch Otorhinolaryngol. 2020. https://doi.org/10.1007/s00405-020-06390-0.
Eissa L, Abdel Razek AAK, Helmy E. Arterial spin labeling and diffusion-weighted MR imaging: Utility in differentiating idiopathic orbital inflammatory pseudotumor from orbital lymphoma. Clin Imaging. 2021;71:63–8.
Razek AAKA. Diffusion tensor imaging in differentiation of residual head and neck squamous cell carcinoma from post-radiation changes. Magn Reson Imaging. 2018;54:84–9.
Abdel Razek AAK. Editorial for “preliminary assessment of intravoxel incoherent motion diffusion-weighted mri [ivim-dwi] metrics in Alzheimer’s Disease.” J Magn Reson Imaging. 2020;52:1827–8.
Kunimatsu N, Kunimatsu A, Miura K, Mori I, Nawano S. Differentiation between solitary fibrous tumors and schwannomas of the head and neck: an apparent diffusion coefficient histogram analysis. Dentomaxillofac Radiol. 2019;48:20180298.
Razek AA, Elsorogy LG, Soliman NY, Nada N. Dynamic susceptibility contrast perfusion MR imaging in distinguishing malignant from benign head and neck tumors: a pilot study. Eur J Radiol. 2011;77:73–9.
Abdel Razek AA, Gaballa G. Role of perfusion magnetic resonance imaging in cervical lymphadenopathy. J Comput Assist Tomogr. 2011;35:21–5.
Abdel Razek AA, Samir S, Ashmalla GA. Characterization of parotid tumors with dynamic susceptibility contrast perfusion-weighted magnetic resonance imaging and diffusion-weighted mr imaging. J Comput Assist Tomogr. 2017;41:131–6.
Razek AAKA. Editorial for “preoperative MRI-based radiomic machine-learning nomogram may accurately distinguish between benign and malignant soft tissue lesions: a two-center study.” J Magn Reson Imaging. 2020;52:883–4.
Acknowledgements
No.
Funding
No.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethics approval
Obtained.
Informed consent
Obtained.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khedr, D., Razek, A.A.K.A. & Talaat, M. Multi-parametric arterial spin labeling and diffusion-weighted imaging of paranasal sinuses masses. Oral Radiol 39, 321–328 (2023). https://doi.org/10.1007/s11282-022-00640-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11282-022-00640-z