Abstract
Campylobacter and Salmonella are the two most prominent foodborne zoonotic pathogens reported in the European Union. As poultry is one of the major sources of these pathogens, it is imperative to mitigate the colonization of these pathogens in poultry. Many strains of lactic acid bacteria (LAB) have demonstrated anti-Salmonella and anti-Campylobacter characteristics to varying degrees and spectrums which are attributed to the production of various metabolites. However, the production of these compounds and consequent antimicrobial properties are highly strain dependent. Therefore, the current study was performed to select a potent LAB and determine its causal attribute in inhibiting Salmonella enterica and Campylobacter jejuni, in-vitro. Six LAB (Lactiplantibacillus plantarum (LP), Lacticaseibacillus casei (LC), Limosilactobacillus reuteri (LR), Lacticaseibacillus rhamnosus (LRh), Leuconostoc mesenteroides (LM) and Pediococcus pentosaceus (PP)) and three serovars of Salmonella enterica (Typhimurium, Enterica and Braenderup) and Campylobacter jejuni were used in the current study. Spot overlays, well diffusion, co-culture and co-aggregation assays against Salmonella and well diffusion assays against Campylobacter jejuni were performed. Organic acid profiling of culture supernatants was performed using HPLC. The results indicated that LRh, LM and PP had the most significant anti-Salmonella effects while LP, LC, LM and PP displayed the most significant anti-Campylobacter effects. Lactic acid and formic acid detected in the culture supernatants seem the most likely source of the anti-Salmonella and anti-Campylobacter effects exhibited by these LAB. In conclusion, Leuconostoc mesenteroides displayed the most significant overall anti-pathogenic effects when compared to the other LAB strains studied, indicating its potential application in-vivo.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Foodborne pathogens are the microorganisms which may transmit to humans via consumption of certain foods (Bintsis 2017). According to the latest reports, Campylobacter and Salmonella are the two most prominent foodborne zoonotic pathogens reported within the European Union (Authority EFS. & European Centre for Disease Prevention and Control 2022). Salmonella is also known as the foodborne pathogen with the highest number of reported human hospitalizations in the United States (Centers for Disease Control and Prevention 2022). Approximately, one million people become sick in the United States each year due to consumption of contaminated poultry products and the Center for Disease Control claims that chicken is one of the major sources of Salmonella and Campylobacter pathogens in humans. On the other hand, as per the estimations published by European Food Safety Association (EFSA) in 2020 updating the 2011 opinion, a 103 reduction of Campylobacter contamination in chicken ceca can cause a 58% reduction of the public health risk (Hazards (BIOHAZ) et al. 2020). Therefore, it is imperative to find solutions to mitigate Salmonella and Campylobacter prevalence in broiler chickens to combat foodborne infections and assure food safety worldwide.
Lactic acid bacteria (LAB) have been intensively studied over the past few decades with the aim of harnessing their antimicrobial properties as alternatives to antibiotics in livestock production. Consequently, many LAB strains have been shown to possess anti-pathogenic effects against Salmonella and Campylobacter and have been used in the food industry due to their antimicrobial food preservation abilities (Reviewed by Vieco-Saiz et al. 2019 and Ibrahim et al. 2021). Furthermore, many LAB strains are identified by the Food and Drug Administration (FDA) under the status of Generally Recognized As Safe (GRAS) and by EFSA under the status of Qualified Presumption of Safety (QPS) and as such, have been used in the food and feed industry for many years (Webb et al. 2022). LAB consist of diverse genera of bacteria which produce different metabolites or compounds which possess antimicrobial properties. Bacteriocins are the one type of antimicrobial compound that are known to be produced by some of the LAB strains. These are antimicrobial peptides with either a broad or narrow spectrum of antimicrobial ability (Wyszyńska and Godlewska 2021). Their mechanisms include disruption of cell wall synthesis and pore formation in cell wall/membrane of pathogens inhibiting their growth and survival (Kumariya et al. 2019). Another important attribute of LAB associated with anti-pathogenic properties, is the production of organic acids (Cizeikiene et al. 2013). Among these organic acids, lactic acid, acetic acid and formic acid, are the major by-products of LAB that are associated with a broad spectrum anti-pathogenic effects. These organic acids create a low intracellular pH environment where pathogens cannot perform their regular metabolic functions such as replication and protein synthesis (Vieco-Saiz et al. 2019). Apart from bacteriocins and organic acids, some LAB can produce hydrogen peroxide, diacetyl, ethanol and carbon dioxide also providing antimicrobial activity against wide range of pathogens (Vieco-Saiz et al. 2019; Wyszyńska and Godlewska 2021; Webb et al. 2022).
Considering the potential of LAB to produce such antimicrobial metabolites against pathogenic bacteria, we selected a number of LAB to screen for the strain with the most broad spectrum of activity in inhibiting different strains of Salmonella and Campylobacter jejuni in broiler chickens. However, the antimicrobial characteristics are highly dependent both on the probiotic and pathogenic strains chosen (Campana et al. 2017). Therefore, in-vitro selection of LAB strains for antimicrobial applications in livestock production required specific focus on certain LAB strains. Accordingly, six commercial LAB strains (homofermentative, obligatory heterofermentative and facultative heterofermentative) belonging to different genera, were chosen for screening against strains of Salmonella enterica and Campylobacter jejuni under in-vitro conditions.
Materials and methods
Bacterial strains
Six LAB strains (which are currently commercially used in multi-strain probiotic supplements for swine and poultry and produced by JHJ Sp Z.o.o, Nowa Wieś, Poland) were selected for anti-pathogenic screening. All the LAB strains had been identified using 16s rRNA sequencing and deposited at the Polish collection of Microorganisms located in Wrocław. The pathogens used in the study included three serovars of Salmonella enterica subspecies Enterica and one strain of Campylobacter jejuni (Table 1).
Anti-Salmonella assays
Spot overlay assays
LAB were inoculated into MRS broth (BD 288130) and incubated aerobically at 37°C for 20 h. Five microliters of each LAB culture were spotted into a labelled MRS agar plate allowed to air dry. These plates were incubated at 37°C overnight. Fifteen microliters of cultures of each Salmonella strain (incubated at 37°C for 16 h in BHI broth (1.10493 Merck)) was added to 30 ml of BHI molten cooled (at 50°C) agar (0.75%) and mixed gently. The Salmonella inoculated agar was overlaid the plate containing LAB spots grown overnight and was further incubated at 37°C overnight. The zone of inhibition surrounding the LAB spots were measured in mm (Four measurements of the radius were taken perpendicularly and averaged). The experiment was performed in triplicate. The three most promising LAB which displayed highest inhibition of all three Salmonella strains were selected for further assays.
Well diffusion assays (WDAs) against Salmonella Typhimurium
The overnight cultures of the selected strains were prepared as described in spot overlay assays section. These cultures were centrifuged at 4000 g for 15 min at 4°C and the supernatant was retained. The pH of the cultures (grown for 20 h) was determined using a pH meter. Supernatant obtained from each culture was neutralized using 1 M NaOH or 1 M HCL, to pH 7 ± 0.2. Untreated and pH neutralized supernantants were filter sterilized using 0.22 μm syringe filters.
Salmonella Typhimurium (DPC6463) overnight culture was prepared as described in spot overlay assays section and 25 µl of the culture was inoculated in 50 ml of BHI molten cooled (at 50°C) agar (1%) and was mixed gently. The inoculated molten agar was poured into a square petri dish and allowed to set for 20 min. Wells of approximately 7 mm in diameter were created in the inoculated agar aseptically, using a sterile pipette tip (1000 µl). Each well was labelled with the names of LAB and 100 µl of the filtered LAB culture supernatants (neat and pH neutralized) was added into the respective wells. For the WDA with neat LAB supernatants, MRS broth (pH = 4) was used as a negative control. The wells were dried at room temperature in a laminar flow hood to the point that when moved to the incubator, the liquid in the wells was not displaced (approximately 30 min). Then the plates were incubated at 37°C for 16 h. Inhibition around the wells were observed and recorded (in mm). The experiment was performed in triplicate.
Co-culture assays
The three LAB which exhibited the strongest inhibition of all three Salmonella strains were selected for co-culture experiments. Double strength BHI broth (for Salmonella) and MRS broth (for LAB) were prepared. Double strength MRS was mixed in equal volume with double strength BHI for the co-culture experiment of LAB with Salmonella. The mixture of double strength media (10 ml) was inoculated with 100 µl of each LAB overnight culture (incubated for 20 h) and 100 µl of Salmonella Typhimurium culture (incubated for 16 h) and incubated for 24 h at 37°C. Selective enumeration of Salmonella Typhimurium in each coculture was performed at 0, 5, 10 and 24 h time points using spot plate method on Salmonella chromogen selective agar (CM1007). Results were graphed to visualize the growth of Salmonella in presence and absence of LAB. The experiment was performed in triplicate. The pH of the cultures was also recorded at each time point.
Co-aggregation assay with Salmonella Typhimurium
The co-aggregation ability of a bacterium is an indicator of the potential inhibition of the colonization of a pathogen in the gut by a beneficial bacteria which co-aggregates with it. Therefore, the co-aggregation ability of the three LAB selected was tested together with Salmonella Typhimurium. All bacterial overnight cultures were prepared as described in the spot overlay assays section. Cultures were centrifuged at 4000 g for 15 min at 4°C. The supernatant was discarded and cell pellet was washed with sterile PBS twice. Then the cell pellet was re-suspended in PBS to a concentration of 0.5 optical density at 600 nm (OD600). OD600 measurements were obtained using BioTek Synergy HT microplate reader. Five hundred microliters of each bacterial suspension was aliquoted into a sterile flat bottom 48 well microtiter plate. Additionally, 250 µl of each LAB suspension was added with 250 µl of Salmonella suspension into the wells of the same plate and mixed by pipetting. The plate was then incubated at 37°C for 24 h. The OD600 reading of the wells was recorded using the microplate reader without shaking the plate. These experiments were performed in triplicate. The co-aggregation ability of each LAB was determined using the following formula (Balakrishna 2013).
Where;
Am = OD600 of mixture of LAB and Salmonella suspensions.
Al = OD600 of LAB suspension alone.
As = OD600 of Salmonella suspension alone.
Anti-Campylobacter assays
Well diffusion assays against Campylobacter jejuni.
Campylobacter jejuni was inoculated in Mueller Hinton broth (BD 275730) supplemented with Campylobacter selective supplement (Skirrow) (SR0069E) according the manufacturer’s directions. After incubating the inoculated broth at 42°C for 48 h under microaerophilic conditions (5% O2, 10% CO2 and 85% N2) using CampyGen™ 2.5 L Sachet (CN0025A, Oxoid), Mueller Hinton agar (1.5%) plates (90 mm circular plates) were spread with 100 µl of this culture and were allowed to dry. Then, using a sterile 200 µl pipette tip, wells of approximately 5 mm in diameter were created aseptically in the agar. The LAB culture supernatants (both neat and pH neutralized) were added to each well (50 µl/well) and then the plates were left for approximately 30 min until the supernatants were absorbed into agar (wells were empty). These plates were incubated at 42°C for 24 h under microaerophilic conditions for 24 h. The inhibition zone around the wells was observed and recorded (in mm). The experiment was performed in triplicate.
Organic acids characterization in culture supernatants
The culture supernatants (after 18 h of incubation) were filtered using 0.22 μm syringe filters. Levels of organic acid metabolites were then quantified by HPLC using a Waters Alliance Separations module e2695 coupled to a Waters 2414 refractive index (RI) detector (Waters, Milford MA, USA). Samples or standards at a volume of 20 µl were injected on to a Rezex Organic acids H + column (300 × 7.8 mm) operated at 60°C. The samples were eluted with H2SO4 (0.005 N) at a flow rate of 0.6 mL/min. Sample detection was performed by comparing retention times of standards. Analytical grade acetic acid, butyric acid, citric acid, lactic acid, formic acid and propionic acid supplied by Merck were used as standards. The assay was performed in duplicate.
Statistical analysis of the data
The measurements from triplicate assays were used to perform ANOVA followed by Tukey’s HSD mean comparison test using Statistica software (Version 14.0.0.15) to identify statistically significant differences among the means.
Results
Anti-Salmonella
Spot overlay assays
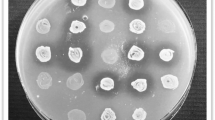
The results of the spot overlay assays indicated that five out of six LAB strains studied (except L. reuteri) are more effective against all three Salmonella serovars (Fig. 1). The highest overall anti-Salmonella activity was observed for L. rhamnosus, L. mesenteroides and P. pentosaceus. Therefore, these three LAB were used for further anti-Salmonella assays.
Well diffusion assays (WDAs)
The pH of the culture supernatants obtained from the six LAB was approximately 4 (L. plantarum- 3.9, L. casei- 3.9, L. reuteri- 4, L. rhamnosus- 4, L. mesenteroides- 4.1 and P. pentosaceus- 4). In order to determine whether the inhibition observed by LAB in spot overlays was due to pH effect (via organic acid production), the WDAs against Salmonella Typhimurium were performed with neat (un-treated) and pH neutralized (pH 7 ± 0.2) culture supernatants of the three LAB selected. Interestingly, no inhibition was observed with the LAB supernatants when pH was neutralized indicating that anti-Salmonella effects observed are possibly due to pH effect/action of organic acids produced by the LAB. The neat supernatants however, displayed inhibition of Salmonella Typhimurium similar to MRS broth at pH 4 (Fig. 2). Therefore, it can be suggested that the.
Radius of inhibitory zone (mm) observed in well diffusion assays (with neat supernatants) against Salmonella Typhimurium. LRh: L. rhamnosus, LM: L. mesenteroides, PP: P. pentosaceus Error bars: ±SD. Homogenous means have been indicated by similar letters identified by Tukey’s HSD test (p value < 0.05)
Co-culture assays of LAB with Salmonella
The results of co-culture assay indicated that the three LAB strains selected (L. rhamnosus, L. mesenteroides and P. pentosaceus) based on promising inhibition observed with spot overlay assays, are equally efficient in inhibiting Salmonella Typhimurium. The number of colony forming units (CFUs) of Salmonella Typhimurium observed for in the presence of LAB was significantly lower when compared to the number of CFUs in the control medium (Fig. 3). Intriguingly, no colonies of Salmonella Typhimurium were present after plating the co-culture at 24 h indicating a complete eradication of Salmonella Typhimurium by LAB. These results suggest that the selected LAB strains possess both bacteriostatic and bactericidal properties against Salmonella Typhimurium.
Selective enumeration of Salmonella Typhimurium in co-culture. A: Comparison of growth of Salmonella with and without LABs. B: Comparison of growth of Salmonella in co-culture with different LAB. C-MRS: Control media (MRS + BHI), LRh: L. rhamnosus, LM: L. mesenteroides, PP: P. pentosaceus. Error bars: ±SD. Homogenous means indicated by similar letters: Tukey’s HSD test (p value < 0.05)
The pH of the co-cultures was measured over time (Fig. 4). It was observed that the pH of C-MRS (double strength BHI + MRS media) inoculated only with Salmonella Typhimurium gradually dropped to approximately 6 at the end of 24 h of culturing. However, co-culture with LAB strains decreased the pH to approximately 4.7 within first 10 h and remained constant until the end of 24 h. This result also supports the assumed role of organic acids produced by LAB in bactericidal effects on Salmonella Typhimurium.
Co-aggregation assays of LAB with Salmonella
The co-aggregation assay was performed with the three most promising LAB strains (L. rhamnosus, L. mesenteroides and P. pentosaceus) together with Salmonella Typhimurium. The results (Fig. 5) indicated that highest co-aggregation is observed with L. mesenteroides.
Anti-Campylobacter well diffusion assays (WDAs)
WDAs against Campylobacter was performed with LAB culture supernatants (neat and pH neutralized). The results indicated that L. mesenteroides, P. pentosaceus and L. casei, followed by L. plantarum displayed the highest inhibition of Campylobacter jejuni (Fig. 6). Similar to anti-Salmonella WDAs, no inhibition was observed with pH neutralized supernatants as opposed to the clear inhibitions observed with neat supernatants (Fig. 7) indicating a potential role of organic acids in anti-Campylobacter activity also.
Organic acid characterization in culture supernatants
The quantification of the organic acids in the culture supernatants is shown in Fig. 8. Propionic or citric acid production was not detected in any supernatants tested. There was significant acetic acid and butyric acid production in the L. reuteri while L. plantarum displayed a limited acetic acid production. Other LAB did not display significant production of these two organic acids. On the other hand, lactic acid and formic acid were found at high levels in the LAB strains which displayed highest anti-Salmonella and anti-Campylobacter properties. Limited inhibition of the pathogens was observed by L. reuteri while the least lactic acid and formic acid production was observed for the same strain. These results suggest a possible role for lactic and formic acids in the anti-Salmonella and anti-Campylobacter properties of the LAB studied.
Discussion
Lactic acid bacteria (LAB) are a group of beneficial bacteria that have earned a reputation in inhibiting pathogens both in-vitro and in-vivo (Ibrahim et al. 2021). It is imperative to select a LAB strain which displays preferably a broad spectrum anti-pathogenic potential for applications to improve the gut health of livestock. The six LAB species that were assessed in the current study are used in multi-strain commercial probiotic supplements for poultry (JHJ Sp. z o.o. 2021) and this product displayed promising results in reduction of Salmonella enteritidis (Smialek et al. 2019) and Campylobacter spp. (Smialek et al. 2018) in broiler gastrointestinal tract (GIT). The current study evaluated the potential of individual LAB strains against three serovars of Salmonella enterica and Campylobacter jejuni in terms of bacteriostatic, bactericidal or co-aggregating properties along with their mode of action. Lecuconostoc mesenteroides has been identified as the most promising candidate LAB due to its anti-Salmonella and anti-Campylobacter activity. Moreover, the results of the current study demonstrated a significant role for lactic and formic acid production in this antimicrobial activity.
As the inhibition ability was lost when the culture supernatants of the strains used in the current study, were pH neutralized, the anti-Salmonella and anti-Campylobacter activity is likely to be associated with a pH effect. LAB are known to impart a pH lowering effect via producing different types of organic acids. Generally, the organic acids demonstrate a non-specific mode of action and thus a broad spectrum antimicrobial activity (Khan et al. 2022). The undissociated form of the organic acids are able to diffuse into the bacterial cells due to its lipophilic nature. Inside the cytoplasm, they dissociate to release H+ ions and reduce the intra-cytoplasmic pH of these pathogens. This eventually results in compromised metabolic functions accounted for bacteriostasis or bactericidal activity. Therefore, organic acids produced by LAB seems to be the likely cause for the strains observed inhibitory effects in the current study. Previous studies reported cases where the anti-pathogenic effects from different LAB strains were maintained (De Giani et al. 2019), decreased (Keeratikunakorn et al. 2023) and disappeared (Ołdak et al. 2020), when pH of the cell free supernatant was neutralized. These studies claim that when the antimicrobial activity is maintained, the inhibitory activity is not due to a pH/organic acid effect whereas decreased or no inhibitory activity is partially or completely due to the effects of pH/organic acid production, respectively. These claims are in agreement with our hypothesis that the inhibition observed by our LAB strains is likely to be due to organic acid production.
Further supporting this assumption, interestingly, different degrees of inhibition were observed for the cultures despite having similar pH. This possibly highlights the significance of specific organic acids produced by each LAB which may display different antimicrobial potential at the same pH. According to our results L. reuteri displayed almost similar pH to L. mesenteroides but displayed much less inhibition of all pathogens studied. It was clear that formic acid and lactic acid content were lowest in the culture supernatant of L. reuteri while L. mesenteroides displayed great production of these organic acids. Similarly, L. reuteri displayed higher production of acetic and butyric acids compared to other LAB studied. Burin et al. (2014) claimed that pathogen inhibition by acetic acid may be higher than lactic acid due to its lower dissociation ability compared to that of lactic acid. The current results suggest that the strains which produce greater amounts of both lactic acid and formic acid appear to cause more inhibition of pathogens compared to L. reuteri which produces acetic acid (which is more effective) but production is lower. It might also be possible that the observed antimicrobial properties are due to a synergistic effect of combinations of organic acids (produced by these LAB) as previously documented by Peh et al. (2020) against Campylobacter species. These authors observed a synergistic potential of caprylic acid, sorbic acid and caproic acid in inhibiting Campylobacter jejuni and Campylobacter coli, in-vitro.
LAB ferment sugars yielding mainly lactic acid to produce the energy necessary for their metabolism. Interestingly, LAB consist of diverse species belong to different genera including Lactobacillus (recently reclassified in to 25 genera such as Lactiplantibacillus, Lacticaseibacillus, Limosilactobacillus, etc.), Leuconostoc, Pediococcus etc. Although fermentation ability is a common feature of these bacteria, they are broadly divided into two major groups of fermenters namely, homofermentative and heterofermentative bacteria. The sole by-product of homofermentation is considered to be lactic acid while heterofermentation yields several by-products such as lactic acid, carbon dioxide (CO2), ethanol and/or acetic acid (Kim et al. 2022). Theoretically, the homofermenters produce 2 moles of lactic acid per 1 mol of glucose while heterofermenters produce less (1 mol) lactic acid per 1 mol of glucose (Kim et al. 2022). Therefore, it is indicative that these differences in fermentation metabolism may attribute to differences in organic acids and their quantities produced by the LAB in the current study. Interestingly, the six LAB were belonged to different fermentation groups. P. pentosaceus is considered more a homofermenter while the rest are obligate (L. reuteri) and facultative (L. plantarum, L. casei. L. rhamnosus and L. mesenteroides) heterofermenters. Therefore, another possibility that these strains displayed different degree of inhibition at the same pH, may be because of other metabolites produced such as ethanol or carbon dioxide production along with the organic acids.
Moreover, different strains from the same LAB species that were used in the current study, are known to produce bacteriocins such as Plantaricin which is produced by L. plantarum (Gong et al. 2010; Kumar et al. 2016), Pediocin by P. pentosaceus (Khorshidian et al. 2021), Caseicin by L. casei (Rammelsberg et al. 1990), Rhamnocin by L. rhamnosus (Jeong and Moon 2015), Mesenterocin from L. mesenteroides (Daba et al. 1991) etc. It is also important to highlight that certain LAB produce bacteriocins which lose their antimicrobial activity in neutral or alkaline pH conditions (Peng et al. 2023). Moreover, Keersmaecker et al. (2006) observed a non-proteinaceous broad spectrum antimicrobial compound which is synthesized by a L. rhamnosus strain and active under lower pH as mediated by accumulation of lactic acid. Therefore, apart from specific fermentation metabolites produced, it is also possible that these LAB displayed different degrees of inhibition due to the production of other proteinaceous or non-proteinaceous antimicrobial compounds which are only active under lower pH. Nevertheless, this theory must be confirmed by further investigation.
In the co-culture, not a single Salmonella colony forming unit (CFU) was observed at 24 h while considerable numbers of Salmonella were present after 10 h incubation. Nevertheless, the pH of both time points was similar. Thus, we suggest that although the pH was similar at both time points (by production of organic acids) it might take some time for the organic acids to diffuse into pathogenic cells, and interfere with the metabolic functions of Salmonella to completely eradicate them from the co-culture. However, another consideration is that these LABs produce a strong antimicrobial metabolite which can eradicate Salmonella, later in their exponential growth which might be activated at a lower pH as previously observed by Keersmaecker et al. (2006).
Moreover, the co-aggregation ability of a probiotic with a pathogen, is a good indication of in-vivo inhibition of pathogen colonization in the GIT. If a probiotic is able to co-aggregate with a pathogen, it is an advantage for the probiotic to release the antimicrobial compounds at a close proximity to these pathogenic bacteria preventing their colonization in the gut (Tuo et al. 2013). Therefore, L. mesenteroides, from our results displays the most promise to combat Salmonella Typhimuriumcolonization in the GIT of broiler chickens.
Conclusion
Among the different strains of different genera belonging to lactic acid bacteria studied, Leuconostoc mesenteroides displayed the most significant overall anti-pathogenic properties against all the food borne pathogens used suggesting its potential for in-vivo applications to combat foodborne pathogens in broiler chickens.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Authority EFS, European Centre for Disease Prevention and Control (2022) The European Union One Health 2021 Zoonoses Report. EFSA J 20(12):e07666. https://doi.org/10.2903/j.efsa.2022.7666
Balakrishna A (2013) In vitro evaluation of adhesion and aggregation abilities of four potential probiotic strains isolated from Guppy (Poecilia reticulata). Braz Arch Biol Technol 56(5):793–800. https://doi.org/10.1590/S1516-89132013000500010
Bintsis T (2017) Foodborne pathogens. AIMS Microbiol 3(3):529–563. https://doi.org/10.3934/microbiol.2017.3.529
Burin RCK, Silva A, Nero LA (2014) Influence of lactic acid and acetic acid on Salmonella Spp. Growth and expression of acid tolerance-related genes. Food Res Int 64:726–732. https://doi.org/10.1016/j.foodres.2014.08.019
Campana R, van Hemert S, Baffone W (2017) Strain-specific probiotic properties of lactic acid bacteria and their interference with human intestinal pathogens invasion. Gut Pathog 9:12. https://doi.org/10.1186/s13099-017-0162-4
Centers for Disease Control and Prevention (2022) Chicken and Food Poisoning. https://www.cdc.gov/foodsafety/chicken.html. Accessed 31 October 2022
Cizeikiene D, Juodeikiene G, Paskevicius A, Bartkiene E (2013) Antimicrobial activity of lactic acid bacteria against pathogenic and spoilage microorganism isolated from food and their control in wheat bread. Food Control 31(2):539–545. https://doi.org/10.1016/j.foodcont.2012.12.004
Daba H, Pandian S, Gosselin JF, Simard RE, Huang J, Lacroix C (1991) Detection and activity of a bacteriocin produced by Leuconostoc mesenteroides. Appl Environ Microbiol 57(12):3450–3455. https://doi.org/10.1128/aem.57.12.3450-3455.1991
De Giani A, Bovio F, Forcella M, Fusi P, Sello G, Di Gennaro P (2019) Identification of a bacteriocin-like compound from Lactobacillus plantarum with antimicrobial activity and effects on normal and cancerogenic human intestinal cells. AMB Express 9(1):88. https://doi.org/10.1186/s13568-019-0813-6
Gong HS, Meng XC, Wang H (2010) Mode of action of plantaricin MG, a bacteriocin active against Salmonella typhimurium. J Basic Microbiol 50(Suppl 1):S37–45. https://doi.org/10.1002/jobm.201000130
Hazards (BIOHAZ) E. P. on, Koutsoumanis B, Allende K, Alvarez-Ordóñez A et al (2020) A Update and review of control options for Campylobacter in broilers at primary production. EFSA J 18(4):e06090. https://doi.org/10.2903/j.efsa.2020.6090
Ibrahim SA, Ayivi RD, Zimmerman T, Siddiqui SA, Altemimi AB, Fidan H, Esatbeyoglu T, Bakhshayesh RV (2021) Lactic acid Bacteria as Antimicrobial agents: Food Safety and Microbial Food Spoilage Prevention. Foods 10(12):Article. https://doi.org/10.3390/foods10123131
Jeong YJ, Moon GS (2015) Antilisterial Bacteriocin from Lactobacillus rhamnosus CJNU 0519 presenting a narrow Antimicrobial Spectrum. Korean J Food Sci Anim Resource 35(1):137–142. https://doi.org/10.5851/kosfa.2015.35.1.137
JHJ Sp. z o.o (2021) Lavipan. http://jhj.pl/files/karty_prod/Ulotki_2021/jhj-lavipan-2021-pl_A4.pdf. Accessed 14 July 2023
Keeratikunakorn K, Kaewchomphunuch T, Kaeoket K, Ngamwongsatit N (2023) Antimicrobial activity of cell free supernatants from probiotics inhibits against pathogenic bacteria isolated from fresh boar semen. Sci Rep 13(1). https://doi.org/10.1038/s41598-023-33062-w. Article 1
Keersmaecker SCJD, Verhoeven TLA, Desair J, Marchal K, Vanderleyden J, Nagy I (2006) Strong antimicrobial activity of Lactobacillus rhamnosus GG against Salmonella typhimurium is due to accumulation of lactic acid. FEMS Microbiol Lett 259(1):89–96. https://doi.org/10.1111/j.1574-6968.2006.00250.x
Khan RU, Naz S, Raziq F, Qudratullah Q, Khan NA, Laudadio V, Tufarelli V, Ragni M (2022) Prospects of organic acids as safe alternative to antibiotics in broiler chickens diet. Environ Sci Pollut Res 29(22):32594–32604. https://doi.org/10.1007/s11356-022-19241-8
Khorshidian N, Khanniri E, Mohammadi M, Mortazavian AM, Yousefi M (2021) Antibacterial activity of Pediocin and pediocin-producing Bacteria against Listeria monocytogenes in Meat products. Front Microbiol 12:709959. https://doi.org/10.3389/fmicb.2021.709959
Kim J, Kim YM, Lebaka VR, Wee YJ (2022) Lactic acid for Green Chemical Industry: recent advances in and future prospects for Production Technology, Recovery, and applications. Fermentation 8(11):Article11. https://doi.org/10.3390/fermentation8110609
Kumar V, Sheoran P, Gupta A, Yadav J, Tiwari SK (2016) Antibacterial property of bacteriocin produced by Lactobacillus plantarum LD4 isolated from a fermented food. Ann Microbiol 66(4). https://doi.org/10.1007/s13213-016-1230-6. Article 4
Kumariya R, Garsa AK, Rajput YS, Sood SK, Akhtar N, Patel S (2019) Bacteriocins: classification, synthesis, mechanism of action and resistance development in food spoilage causing bacteria. Microb Pathog 128:171–177. https://doi.org/10.1016/j.micpath.2019.01.002
Ołdak A, Zielińska D, Łepecka A, Długosz E, Kołożyn-Krajewska D (2020) Lactobacillus plantarum strains isolated from Polish Regional cheeses exhibit anti-staphylococcal activity and selected Probiotic properties. Probiotics Antimicrob Proteins 12(3):1025–1038. https://doi.org/10.1007/s12602-019-09587-w
Peh E, Kittler S, Reich F, Kehrenberg C (2020) Antimicrobial activity of organic acids against Campylobacter Spp. And development of combinations—A synergistic effect? PLoS ONE 15(9):e0239312. https://doi.org/10.1371/journal.pone.0239312
Peng Z, Xu X, Fan P, Qiao B, Xie M, Huang T, Xiong T (2023) Identification and characterization of a novel pH and heat stable bacteriocin-like substance lactococcin036019 with food preserving potential. Food Control 148:109682. https://doi.org/10.1016/j.foodcont.2023.109682
Rammelsberg M, Müller E, Radler F (1990) Caseicin 80: purification and characterization of a new bacteriocin from Lactobacillus casei. Arch Microbiol 154(3):249–252. https://doi.org/10.1007/BF00248963
Smialek M, Burchardt S, Koncicki A (2018) The influence of probiotic supplementation in broiler chickens on population and carcass contamination with Campylobacter spp. - Field study. Res Vet Sci 118:312–316. https://doi.org/10.1016/j.rvsc.2018.03.009
Smialek M, Kaczorek E, Szczucińska E, Burchardt S, Kowalczyk J, Tykałowski B, Koncicki A (2019) Evaluation of Lactobacillus spp. And yeast based probiotic (Lavipan) supplementation for the reduction of Salmonella enteritidis after infection of broiler chickens. Pol J Vet Sci 22(1):5–10. https://doi.org/10.24425/pjvs.2018.125616
Tuo Y, Yu H, Ai L, Wu Z, Guo B, Chen W (2013) Aggregation and adhesion properties of 22 Lactobacillus strains. J Dairy Sci 96(7):4252–4257. https://doi.org/10.3168/jds.2013-6547
Vieco-Saiz N, Belguesmia Y, Raspoet R, Auclair E, Gancel F, Kempf I, Drider D (2019) Benefits and inputs from lactic acid Bacteria and their bacteriocins as Alternatives to Antibiotic Growth promoters during Food-Animal production. https://doi.org/10.3389/fmicb.2019.00057. Front Microbiol 10
Webb L, Ma L, Lu X (2022) Impact of lactic acid bacteria on the control of Listeria monocytogenes in ready-to-eat foods. Food Qual Saf 6:1–11. https://doi.org/10.1093/fqsafe/fyac045
Wyszyńska AK, Godlewska R (2021) Lactic acid Bacteria – A Promising Tool for Controlling Chicken Campylobacter infection. Front Microbiol 12:703441. https://doi.org/10.3389/fmicb.2021.703441
Acknowledgements
We acknowledge JHJ sp z o.o., Poland for providing LAB for our experiment. We also thank APC Microbiome, Ireland and Salmonella National Reference Laboratory, DAFM, Ireland for providing Salmonella Typhimurium and Salmonella Braenderup strains, respectively and Aarhus University, Denmark for providing Campylobacter jejuni strain used in the current experiment. We specially thank Helen Slattery from Teagasc Food Research Center, Ireland for performing the HPLC for organic acid characterization.
Funding
This research was carried out under the funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 955374.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Ramesha N. Wishna-Kadawarage. The first draft of the manuscript was written by Ramesha N. Wishna-Kadawarage. Funding acquisition, supervision, review and editing was by Rita M. Hickey and Maria Siwek. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wishna-Kadawarage, R.N., Hickey, R.M. & Siwek, M. In-vitro selection of lactic acid bacteria to combat Salmonella enterica and Campylobacter jejuni in broiler chickens. World J Microbiol Biotechnol 40, 133 (2024). https://doi.org/10.1007/s11274-024-03946-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-024-03946-8