Abstract
The aim of the present study was to analyze the response to exposure to pollutants (trace elements and organic pollutants) using biomarkers (micronucleated cells and glutathione S-transferase and catalase activity) in uçá crab Ucides cordatus. The study was carried out at four sites: Cacha Prego (CP) and Ponta Grossa (PG), areas with low anthropic activity; and Ilha de Maré (IM) and Pitinga (PT), areas affected by industrial activity. At each site, soil and crab samples were collected to analyze the contents of potentially toxic elements (total concentration and chemical partitioning of trace elements), polycyclic aromatic hydrocarbons (PAHs), and polychlorinated biphenyls (PCBs). Both total concentrations and geochemical fractions of Cu, Zn, and Ni in soils were significantly higher in IM. Likewise, higher reactive Pb contents were observed in mangroves both in IM and in PG. Values above quality limits were observed for organic pollutants (PAHs) in soils from CP and PT, while PCB contents were below the maximum permissible levels. Metals in crabs also showed spatial variations, with higher Cu concentrations in all tissues in IM and PT and higher Ni concentrations in hepatopancreas in PT during the dry season. PAH values in crabs did not show spatial variations; however, crustaceans with contents above maximum limits in their muscle tissue were found in CP. Crabs from the Baia de Todos Santos showed different responses in biomarker expression, with higher enzymatic activity and greater numbers of micronucleated cells in crabs from IM, suggesting oxidative stress and genotoxicity in this mangrove forest.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Mangrove forests are wetlands associated with the provision of ecosystem services, such as food production for coastal communities, since they constitute breeding and shelter areas for many marine and estuarine animal species (Mcleod and Salm, 2006). In Baía de Todos os Santos (BTS), important artisanal fishing and shellfish gathering grounds are located in mangrove forests, making this ecosystem a relevant environment for animal protein supply and employment generation in coastal communities in the BTS (Souto, 2008; Soares et al., 2009).
The crab Ucides cordatus, commonly known as uçá crab, is one of the main fishing resources in the north and northeastern regions of Brazil and represents one of the main exploited crustacean species in Baía de Todos os Santos (Soares et al., 2009; Pinheiro et al., 2016). In addition to the relevance of its harvesting, U. cordatus plays a major role in mangrove ecosystem functioning, being involved in nutrient recycling and influencing soil biogeochemical processes (Nordhaus et al., 2006, 2009; Araújo-Júnior et al., 2016).
Mangroves in the BTS, mainly in the north and northeast, are threatened by intense anthropogenic activity, including ports, marine terminals, and industrial areas, with ongoing developments in the chemical, metal, and petrochemical industries, which have promoted enrichment of organic pollutants (∑PAH sediments 1–408629 ng g−1, Celino and Queiroz, 2006; PCB bivalves <0.08–50.1 ng g−1, Santos et al., 2020) and trace elements (Cu sediments 2.7–38.9 mg kg−1, Zn sediment 9.4–212 mg kg−1, Pb 5–125 mg kg−1, Rocha et al., 2012; Cu sediments 122±3.4 mg kg−1, Andrade et al., 2017; Cu sediments: 92.7–97.5 mg kg−1, Cr sediments 56.2–66.0 mg kg−1, Santos et al., 2021) in coastal ecosystems. The presence of these pollutants has compromised the quality of these environments, as well as fishing and shellfish gathering activities (Carvalho, 2020; Carvalho and Vidal, 2020), and poses a risk both for the biota and for the human species, taking into account the bioaccumulation and trophic transfer potential shown by these pollutants (Hatje et al., 2006; Santos et al., 2021).
Environmental quality has been monitored using different types of biomarkers present in aquatic organisms worldwide (Jesus et al., 2021; Yamamoto et al., 2023; Rao et al., 2023; Truchet, et al., 2023). Biomarkers are defined as any biological alterations that can be used to detect and predict the effects of pollutants on the environment. These responses include alterations in molecules, cells, tissues, physiological mechanisms, and animal behavior (Van der Oost et al., 2003; Walker et al., 2010). Thus, the use of suitable biomarkers is essential to more accurately assess the risks of exposure and the consequent implications for their mitigation and the protection of the animal’s integrity (Kroon et al., 2017; Yamamoto et al., 2023). Biochemical biomarkers, such as catalase and glutathione S-transferase enzyme activities, provide an early warning system able to demonstrate the initial effects caused by exposure to xenobiotic compounds (Lam, 2009, de Oliveira et al., 2024). Moreover, glutathione S-transferase (GST) and catalase (CAT) activity in Ucides cordatus has been linked to the presence of metals in their environment (Pinheiro et al., 2012; Duarte et al., 2014, 2016; Banci et al., 2017; Silva et al., 2018; Santos et al., 2019). Thus, the use of biochemical biomarker responses may constitute a major tool to indicate exposure of organisms to xenobiotic compounds.
Taking into account the importance of the exploitation of U. cordatus in this bay and its role in the biogeochemical processes that take place in mangrove forests, this study aimed to analyze the contents of trace elements, polycyclic aromatic hydrocarbons (PAHs), and polychlorinated biphenyls (PCBs) in soils and in tissues of this crustacean, as well as assessing this species’ responses to exposure using genetic and biochemical biomarkers.
2 Material and Methods
2.1 Study Area
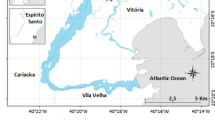
The study was carried out in four mangrove forests in Baía de Todos os Santos (BTS, Fig. 1), two of them located at the entrance of the bay, in Itaparica Island (Cacha Prego, CP, and Ponta Grossa, PG), both areas considered to have a low anthropic impact and low pollution; one forest located in the northeast region (Ilha de Maré, IM), close to industrial areas and maritime ports, and one located in the northern region (Pitinga, PT), in the Subaé River estuary, an area with a history of metal pollution associated with an abandoned metallurgical factory (Hatje et al., 2006, 2009; Rocha et al., 2012). These sites were selected to cover different sections of the BTS, analyzing potential spatial variations according to the higher or lower exposure to anthropogenic stressors both to establish baseline values and to better understand soil-to-biota transfer processes.
2.2 Sample Collection
Soil samples were collected at low tide during the dry season (DS), between December 2020 and February 2021, and during the rainy season (WS), between June and July 2021. At each site and during each season, 12 pooled samples were collected from the surface soil layer (S, 0–5 cm) and 12 more samples were collected from the deep soil later (D, 15–30 cm); each sample was composed of three subsamples and was collected using stainless steel corers. Additionally, 12 male Ucides cordatus specimens were collected (carapace length >60 mm) at each mangrove site during the dry season in 2019, during the dry season in 2020/2021, and during the rainy season in 2021. Moreover, 12 male U. cordatus specimens to be used for enzymatic biomarker analyses were collected in 2022 in the Marine Extractive Reserve of Baía do Iguape (Resex-Iguape), located in a gorge in the Paraguaçu river, in a low-pollution area far away from the more industrialized areas within Baía de Todos os Santos (Andrade et al., 2017) and therefore established as a reference site for this study.
2.3 Sample Analysis
2.3.1 Soil Analysis
Soil samples were characterized in terms of pH and Eh using a HI8424 portable pH-meter (Hanna Instruments). The pH electrode used for the analysis was previously calibrated using pH 4 and pH 7 standards, while the Eh-meter was tested using a 220-mV standard redox solution (Hach Be right). Texture was determined by sieving soil through 2-mm and 0.053-mm mesh sizes to determine the proportions of sand and fine fraction (silt and clay). Total organic carbon content (TOC) was also determined using an elemental analyzer (FlashEA1112, ThermoFinnigan) on dry soil samples. For this analysis, all samples had been previously subjected to acid attack using 10 ml HCl (1N) to remove carbonates (Schumacher, 2002). After adding the acid solution, samples were shaken for 1 h, then centrifuged to remove HCl, washed five times in deionized water, and dried in an oven at 40 °C.
Total content of Fe, as well as of elements Cu, Zn, Ni, Cd, Pb, and Cr, was analyzed in soils after sample digestion using 9 ml ultrapure nitric acid (HNO3 65%) and 3 ml ultrapure hydrochloric acid (HCl 37.5%) in a closed system in a closed-vessel microwave system (Milestone; ETHOS EASY).
As for the extraction of geochemical forms of metals, two fractions were considered: the reactive fraction and the oxidizable fraction. The reactive fraction was considered to be the sum of the exchangeable metal fraction and metals associated with carbonates and with oxyhydroxides (both crystalline and amorphous), while the oxidizable fraction includes metals associated with organic matter and with the pyritized fraction (Huerta-Díaz and Morse, 1992).
To extract both fractions, the BCR method (Rauret et al., 1998) was followed in combination with the extraction method proposed by Otero et al. (2009). The reactive fraction was extracted according to the following steps:
-
F1, soluble fraction, exchangeable, and associated with carbonates (ExCa): 30 ml acetic acid (0.11 mol l−1; pH = 4.5) was added to each sample (2 g wet sample); samples were then shaken at 25 °C for 16 h. After shaking, samples were centrifuged to remove the supernatant (fraction 1), washed in ultrapure water, and then centrifuged again, a procedure that was repeated before each subsequent stage.
-
F2, fraction associated with amorphous iron oxides (Am): to the residue of the previous fraction, 20 ml of solution containing 20 g ascorbic acid + 50 g sodium citrate + 50 g bicarbonate + 1 l ultrapure H2O and N2(g) at pH 8 were added. Samples with the solution were shaken at 25 °C for 24 h and were subsequently centrifuged to collect the washed extract.
-
F3, fraction associated with crystalline iron oxyhydroxides (Cr): each sample was added 20 ml of solution containing 73.925 g sodium citrate + 9.24 g NaHCO3 in 1 l ultrapure H2O and 3 g sodium dithionite, then shaken for 30 min at 75 °C, and then centrifuged to collect the washed extract.
-
F4, reduced forms associated with organic matter and oxidizable sulfides (Red): each sample was added 10 ml H2O2 (8.8 M) and warmed in a bath at 85 °C until evaporation down to 3 ml, followed by a second addition of 10 ml H2O2. Samples were kept warm (in a bath at 85 °C) until evaporation down to 1 ml, at which point 50 ml of AcNH4 solution was added and samples were shaken for 16 h at 25 °C.
Considering the distribution of each metal in the reactive and oxidizable fraction, the degree of pyritization of Fe (DOP; Berner, 1984) and of trace metals (DTMP, Huerta-Díaz and Morse, 1992) was determined, assuming that most of the metal in the oxidizable fraction is found in the form of pyritic metal (Me-pyr).
2.4 Analysis of Trace Metals in Ucides cordatus
To determine levels of trace metals in crustaceans, all U. cordatus specimens were washed in distilled water to remove mud and immediately dissected to collect gills, hepatopancreas, and muscle. The extracted tissues were freeze-dried (LIOTOP L101 Freeze-Drier) for 72 h, ground, and digested on a digestion block with 7 ml ultrapure HNO3 and 2 ml ultrapure H2O2 (Joksimovic and Stankovic, 2012). All analyses were performed on pooled samples, each one of them composed of tissue from three different specimens.
Analytes were determined by atomic absorption spectroscopy (AAS-Perkin Elmer) in all the study matrices, and the analytical method used was validated using certified reference material for soil (SO-3, Canadian Certified Reference Material Project, Canada), leaves (NIST-1547), and animal samples (NIST-1577b, bovine liver), with recovery rates over 79%.
2.5 Analysis of Polycyclic Aromatic Hydrocarbons and Polychlorinated Biphenyls in Soils and in Crab
Soil samples (n=3) and edible U. cordatus tissues (hepatopancreas, n=3, and muscle, n=3) were analyzed to determine the contents of the following polycyclic aromatic hydrocarbons: naphthalene (NAP), acenaphthylene (ACY), acenaphthene (ACP), fluorene (FLR), phenanthrene (PHE), anthracene (ANT), fluoranthene (FLT), pyrene (PYR), benz(a)anthracene (B(a)A), chrysene (ChR), benzo(b,j)fluoranthene (B(b)F + B(j)F), benzo(k)fluoranthene (B(k)F), benzo(a)pyrene (B(a)P), dibenz(a,h)anthracene (DhA), benzo(g,h,i)perylene (B(g,h,i)P), and indeno(1,2,3-cd)pyrene (IcP), as well as of polychlorinated biphenyls (PCB 28, 52, 101, 138, 153, 180).
PAH extraction was performed following the EPA 3545 method, while contents were determined by gas chromatography/mass spectrometry (GC-MS) following the EPA 8270C method, with a limit of detection of 0.001 mg kg−1. Samples and extracts were kept until analysis at 3 °C in glass containers previously washed in methanol and acetone. B(a)P, B(a)A, B(b)F, and ChR values were taken into account for the analysis of crab samples, taking into account the specifications in European Commission Regulation no. 835/2011, which considers both the isolated B(a)P value and the sum of the values for B(a)P, B(a)A, B(b)F, and ChR as indicators for the definition of the maximum permissible values for PAHs in foodstuffs. The Regulation defines 2.0 μg g−1 as a maximum threshold for B(a)P and 12.0 μg g−1 for ∑ [B(a)P, B(a)A, B(b)F, ChR] in meat (muscle) from smoked crab appendages, with no defined limits for hepatopancreas.
For PCB’s analysis, soil (n=1) and animal tissue (n=1) samples were doped with carbon-13-labeled toxic isomers and subsequently left to rest for 3 h. Soxhlet extraction with toluene was performed for 8 h until evaporation, followed by redissolution in hexane. Samples were later treated with acid and separated to concentrate the organic phase in a rotary evaporator until an approximate volume of 5 ml was reached. Purification was immediately performed using automated chromatography equipment. Detection was performed by high-resolution electron ionization spectrometry (Thermo Scientific DFS). The analysis procedure was based on the EPA method 1668C. PAH and PCB analyses were performed by the Research Support Services (Servizos de Apoio Á Investigación, SAI) of the University of A Coruña.
2.6 Enzymatic Biomarker Analysis
2.6.1 Glutathione S-Transferase and Catalase Activity
GST and CAT activity was analyzed in crabs collected at Ilha de Maré (2020/2021), an impacted mangrove site, and in Santiago do Iguape (2022), the reference site. The collected specimens were transferred into a plastic box containing ice until their metabolic activity was visibly reduced. An incision in the cephalothorax region was then performed using surgical material to extract the hepatopancreas. Hepatopancreas samples were immediately transferred to 1.5-ml cryotubes (Eppendorf®), labeled, and stored at −80 °C in an ultra-low temperature freezer (Sanyo®, Ultra Low) to determine the activity of enzymes CAT and GST. Due to the small amount of biological material, each sample was composed of a pool of hepatopancreas samples from three adult individuals to achieve the minimum mass required for the analysis (0.5 g), according to Mota et al. (2023).
The frozen pooled samples were homogenized in a phosphate-buffered solution (pH=6.8) at a 1:10 proportion (weight:volume) in an ice-cooled container. The homogenized material was then centrifuged at 10000 RPM for 20 min in a centrifuge (Hettich®, MIKRO 220R) refrigerated at 4 °C. After centrifuging, the supernatant was separated using a micropipette and transferred to previously labeled cryotubes. Samples were stored at −80 °C in an ultra-low temperature freezer to later determine the activity of enzymes CAT and GST. CAT activity (nmol H2O2 min−1 mg pt−1) was determined based on exogenous H2O2 degradation to subproducts H2O and O2 (Aebi, 1987). Readings were performed in triplicate using a UV/Vis spectrophotometer (Biochrom Libra®, S21/S22, Reaction kinetics software) in quartz cubes at a wavelength of 240 nm. GST activity (nmol CDNB min−1 mg pt−1) was determined based on the catalysis of the conjugation reaction for substrate 1-26-chloro-2,4-dinitrobenzene (CDNB) with reduced glutathione in acrylic trays at a wavelength of 340 nm (Keen et al., 1976). Total hepatic protein concentration was quantified using a commercial kit (Doles®).
2.7 Genetic Biomarker Analysis (Micronucleus Tests, MN)
Micronucleus tests were performed on crabs collected at Ilha de Maré, an impacted site, and in Cacha Prego, in the reference site, collected in 2020/2021. For the analysis, 1 mL of hemolymph was extracted from each crab by inserting a hypodermic syringe with a needle through the membrane of the carpal-propodal joint of a cheliped. The needle was immediately removed from the syringe, and a drop of hemolymph was deposited onto a histological slide and smeared using a clean slide (smearing slide). Freshly smeared slides were air-dried for 20 min at room temperature and subsequently immersed in Carnoy’s solution (methanol/acetic acid 3:1) for 20 min to fix the hemolymph sample, then air-dried again (Pinheiro et al., 2013).
All the slides were stained by immersion in 2% Giemsa solution in phosphate buffer at pH 6.8 (Na2HPO4+KH2PO4) for 20 min. Stained slides were immediately removed from the staining solution and washed in deionized water (Duarte et al., 2016). Once the slides were completely dry, a drop of Entellan® was deposited on each slide to place and fix a slide cover on the hemolymph smear.
Cell counts and verification of their status (normal or showing nuclear malformations: micronuclei) were performed under an Olympus® binocular microscope (model CX31) with 1000× magnification in immersion oil. Micronucleated cells were identified according to Countryman and Heddle (1976) and Duarte et al. (2016). In total, two slides were mounted for each specimen to guarantee a minimum cell count of 1000 (Pinheiro et al., 2013; Duarte et al., 2016).
2.8 Statistical Analysis
The results were analyzed using descriptive statistics (Sigmaplot 12.0), and a Kruskal-Wallis non-parametric test was applied at a 5% level of significance (XLSTAT 2014) to compare the results obtained from the different study sites. This non-parametric test was selected due to its higher robustness and lower statistical assumption requirements (Reimann et al., 2008).
3 Results
3.1 General Soil Characterization
Ilha de Maré (IM) showed significantly higher silt and clay contents than the remaining mangrove sites (Table 1). pH ranged from 4.5 to 7.7, with mean soil values close to neutrality and without seasonal variations, except for Pitinga (PT), where values were lower in the dry than in the rainy season, ranging from highly acidic to moderately acidic (S, 4.8±0.4; D, 5.9±0.4). Eh during the dry season varied between +92 and +188 mV, with mean values typical of suboxic soils in all the mangroves (Eh 200–300 mV; Table 1) except for IM, where conditions in the deep soil layer were predominantly anoxic. During the rainy season, values varied between +30 and +155 mV, with most of the soils showing values within the suboxic range, the deep layer being predominantly anoxic in IM, PG, and CP (Table 1). Seasonal differences were observed at both soil depths in IM, as well as in the deep layer in PT, with higher values during the dry season (p<0.05). TOC ranged between 0.8% and 6.6%, without seasonal differences but showing significant spatial differences, with higher values in IM during the dry (S, 6.0±0.58%; D, 4.8± 0.3%) and rainy season (S, 5.9±1.0%; D, 4.8±0.4%) than in the remaining areas (0.8–3.9%).
Total Fe ranged from 0.10 to 2.8% and showed the highest concentrations in IM (Table 1), both during the dry (S, 2.3±0.5%; D, 2.6±0.1%) and the rainy season (S, 2.3±0.5%; D, 2.6±0.1%). Contents in CP during the rainy season were also higher than those found in PT and PG at both depth levels (p<0.05). Seasonal differences were only observed for the deep soil layer in IM and CP, where contents were higher during the rainy season. Reactive Fe varied between 128 and 3344 mg kg−1, with clear spatial differences in the two study periods. In the dry season, values in the deep layer in IM (1319–1480 mg kg−1; mean, 1414±84.7 mg kg−1) were significantly higher than those in the remaining areas (267–983 mg kg−1; mean, 661±249 mg kg−1). During the rainy season, contents in the surface layer in IM and CP were higher than those in PG and PT, while values in the deep layer in IM were higher than those found in all other mangrove sites (170–1254 mg kg−1). Reduced Fe contents (85–9233 mg kg−1) also showed significant spatial variations both during the dry season, when values in the deep layer were highest in IM and CP, and during the rainy season, when concentrations at all depths in IM were higher than those observed in all other mangroves (Table 1).
3.2 Pollutants in Soils
3.2.1 Potentially Toxic Metals
Cd values were below the limit of detection in all samples (ld, 0.01 mg l−1). Total Cu ranged from below the limit of detection (<ld–0.02 mg l−1) to 89.4 mg kg−1, with the highest values found in IM during both seasons (Table 2). Non-residual contents (<ld–40.1 mg kg−1) (Table 3) were mainly present in the reduced phase, except during the dry season in PT, where concentrations in this fraction were lower (<ld–0.30 mg kg−1) than reactive contents (<ld–0.90 mg kg−1), although differences were not significant (p>0.05). Non-residual concentrations showed spatial variability only in terms of Cu contents in the reduced phase, which were significantly higher in IM (7.80–40.1 mg kg−1; mean, 25.0±10.5 mg kg−1) (p<0.05).
Total Zn ranged from values <ld (0.02 mg l−1) to 65.7 mg kg−1 and also showed significant spatial variations (p<0.05), with the highest contents found in the IM mangrove site (Table 2). Most of the Zn in IM was found in the potentially bioavailable phase (∑ F1➔F4), while in the remaining mangrove sites, the highest values were found in the residual phase (PT, 57.2±24.9%; CP, 69.6±14.4%; PG, 80.1±9.3%). Non-residual contents varied from values <ld to 32.7 mg kg−1 (Table 3) and were mainly present in the reactive phase, where contents were significantly higher in IM (p<0.05).
Total Cr ranged from values <ld (0.01 mg l−1) to 63.0 mg kg−1 and showed significant spatial variations (p<0.05), with the highest contents found in IM (Table 2). Contents in geochemical fractions in PT were below the limit of detection in all samples. In the remaining mangroves, Cr was mainly present in the residual fraction (IM, >70%; CP, >74%; and PG, >87%), with potentially bioavailable contents showing different distribution patterns among geochemical fractions (Table 3). Concentrations in IM were present both in the reactive (<ld–10.6 mg kg−1) and in the oxidizable phase (<ld–8.60 mg kg−1), while in CP and PG, contents were only detected in the reduced phase (<ld–6.30 mg kg−1) and in the reactive phase (0.20–1.60 mg kg−1), respectively. No significant spatial differences were found (p>0.05).
Total Ni in IM varied between 15.0 and 21.2 mg kg−1, without seasonal variations or vertical differences (p>0.05). In PT, it ranged from below the ld (0.05 mg l−1) to 5.45 mg kg−1, with only two samples showing values above the LD (WS, 5.33±0.20 mg kg−1). In CP and PG, all values were below the limit of detection (Table 2). Ni contents in geochemical fractions were below the limit of detection in PG, PT, and CP in all samples (Table 3), while in IM, non-residual contents accounted for 10.4% and 20.2% or total contents in the dry and rainy season, respectively, detected only in the oxidizable fraction (<ld to 4.20 mg kg−1).
Pb contents during the dry season were only detected in soils from IM (Table 2), where concentrations ranged from 15.6 to 21.5 mg kg−1; meanwhile, in the rainy season, values remained below the limit of detection (0.01 mg l−1; <10 mg kg−1) in the surface soil layer in PT and PG, with the detected concentrations ranging from 10.2 to 26.3 mg kg−1, with no spatial variations (p>0.05). Most of the Pb was present in the residual fraction (>95%), with potentially bioavailable contents detected only in the reactive fraction, ranging from values <ld to 0.70 mg kg−1 (Table 3). Concentrations showed clear spatial differences, with the highest concentrations found in PG during the dry season (in depth) and in PG and IM during the rainy season (in the upper layer) (p<0.05). Metal concentrations, both in total and in the geochemical fractions, did not show significant seasonal variations.
3.2.2 PAH and PCB Concentrations
PAH concentrations in soils varied between values below the limit of detection and 44.7 ng g−1, without clear spatial variations in content except in the case of [B(b+j)F], which showed higher values in PG (19.7±2.86 ng g−1) than in PT (6.61±2.83 ng g−1), and B(a)P, significantly higher in CP (11.7±1.30 ng g−1) than in IM (1.79±0.38 ng g−1) (p<0.05). Values above the threshold effect level (TEL), the minimum level required for the potential risk of toxic effects for the biota, established by National Oceanic and Atmospheric Administration of the United States (NOAA, 1999), were found in samples for [ACY] in CP and PT and for [DhA] also in CP. PCBs ranged from values <0.03 to 4.18 ng g−1, with the highest values observed for congeners PCB101, PCB153, and PCB180 (Table 4), which were higher in mangroves in IM and PG. These areas also showed the highest values for the total sum of all PBCs, which ranged from 9.25 ng g−1 in PT to 16.05 ng g−1 in IM.
3.3 Pollutant Concentrations in Ucides cordatus
3.3.1 Potentially Toxic Trace Elements
Cd contents in U. cordatus remained below the LD for all samples. Cu concentrations ranged from 20.2 to 1320 μg g−1, and it was mainly present in hepatopancreas and gills. Concentrations in tissues showed clear spatial differences in all the study periods and were significantly higher in IM and PT (p<0.05) for all tissues (Fig. 2). Values found in gills (76.8–1320 μg g−1) did not show any seasonal variations, except for IM, where concentrations during the dry season (DS, 918±315 μg g−1) were significantly higher than those in the rainy season (WS, 463±105 μg g−1). Likewise, concentrations in muscle varied between 33.4 μg g−1 and 104 μg g−1, without significant seasonal variations (p>0.05), except for CP, where contents in the dry season were also higher than in the rainy period (p<0.05). Contents in hepatopancreas ranged from 20.2 to 1133 μg g−1, with higher concentrations during the dry season in all areas (p<0.05).
Concentrations of trace elements (Cu, Zn, Cr, Pb, and Ni) in μg g− in tissues (M, muscle; G, gills; and H, hepatopancreas) of Ucides cordatus in mangroves in Baía de Todos os Santos during the dry season (DS) and the rainy season (WS). Different uppercase letters indicate spatial differences within each season, while * indicates seasonal variations within each area. Blank plots indicate that values were below the limit of detection.
Zn contents ranged from 71.1 to 527 μg g−1, and it was mainly present in muscle tissue (Fig. 2). Concentrations in hepatopancreas showed spatial variations only during the dry season, where the highest values were found in mangroves in PT; the same pattern was found for gill tissue, where contents during this period were higher in PT and IM. Muscle tissue showed spatial variation in the two seasons, again with higher concentrations in PT during the dry season; meanwhile, values during the rainy season were higher in crabs from PT, IM, and CP, without significant differences among these areas (p>0.05).
The highest contents in hepatopancreas were found during the dry season for all mangrove sites, while for gills in PT and CP and for muscle in IM and PT, the highest concentrations were found in the rainy season (p<0.05).
Cr concentrations ranged from values <ld to 8.40 μg g−1 and were detected only in gills during the rainy season (p<0.05), with concentrations in PT remaining below the limit of detection for all samples (Fig. 2). Spatial variations were only observed during the rainy season, when values in IM (7.54±0.57 μg g−1) were higher than those found in CP (6.33±0.69 μg g−1). Ni contents in muscle were below the ld for all sites, as were contents in gills in PG and CP during all seasons and in IM and PT during the dry season (Fig. 2). Contents detected in the aforementioned tissue ranged from values <ld to 3.51 μg g−1 and did not show significant spatial differences (p>0.05). Meanwhile, concentrations in hepatopancreas (<ld to 14.6 μg g−1) showed clear spatial variations during the rainy season, with higher contents in PT (10.9±4.38 μg g−1) than in the remaining mangrove sites (p<0.05). All samples collected in the PG mangrove site during the rainy season showed values below the LD.
Pb contents remained below the LD for all samples from CP and PG (Fig. 2). Values above the limit of detection were only found in gills in IM during the dry season (<ld–6.32 μg g−1) and in PT during both seasons (<ld–6.64 μg g−1), without spatial variations but with significant seasonal differences in PT, where contents were highest during the dry season (p<0.05).
3.4 PAH and PCB Concentrations in Ucides cordatus
Contents of the PAH B(a)P in hepatopancreas varied between 0.17 and 21.1 ng g−1 and remained below the limit of quantification (<0.20 ng g−1) in all muscle tissue samples, while values for ∑[B(a)P, B(a)A, B(b)F, ChR] ranged from 3.74 to 48.4 ng g−1 to values <LOQ to 20.1 ng g−1 in hepatopancreas and muscle, respectively (Table 5). Values above the maximum thresholds permitted by the European Union (EU Regulation no. 835/2011) were only observed in muscle samples from CP for the sum ∑[B(a)P, B(a)A, B(b)F, ChR], as shown in Table 5. No significant spatial differences were found (p>0.05).
PCB contents in U. cordatus varied between values <0.03 and 1.32 ng g−1 in hepatopancreas and between <0.03 and 0.20 ng g−1 in muscle tissue (Table 6). The highest values in hepatopancreas were observed for congener PCB153 (IM, CP, PT), followed by PCB52 (PG) and PCB138 (CP and PT), while the highest concentrations in muscle were detected for congeners PCB153, PCB138, and PCB101. In PT, the highest values for PCB153 and PCB138 in both tissues were found, as well as for the sum of all the analyzed PCBs. PG had the highest value for congener PCB52 (0.93 ng g−1) compared with those found in the remaining areas, where concentrations ranged from values <ld to 0.29 ng g−1. The recorded values for the sum of all the analyzed PCBs suggest differences among the sites, with the highest values found in PT both for hepatopancreas and for muscle.
3.5 Biomarkers in Ucides cordatus
GST activity (Fig. 3) in IM showed clear differences among sites and was significantly higher (p<0.05, IM 493 ±17.5 μmol min mg protein−1) than that in the reference site (RESEX 279 ±59.5 μmol min mg protein−1); the same pattern was observed for the CAT enzyme (IM 47.9 ± 9.70 nmol min mg protein−1; RESEX, Santiago do Iguape 11.5 ± 1.40 nmol min mg protein−1). Micronucleated cells ranged from 0.0 to 6.0‰, with significantly higher values in IM (4.0±1.0‰) than in CP (0.8±0.8‰).
Glutathione-S-transferase (GST) and catalase (CAT) activity in hepatopancreas (A, B) and micronucleated cells in hemolymph (C) of Ucides cordatus in mangroves in Ilha de Maré (IM), Cacha Prego (CP), and Santiago de Iguape (control site, CNTRL) in Baía de Todos os Santos. Different letters indicate significant differences (p<0.05). na, not analyzed
4 Discussion
4.1 Geochemistry of Potentially Toxic Metals Related to Their Bioavailability
Soils exhibited spatial differences in terms of total metal contents, with the highest values found in IM, which can be explained by the location of this mangrove forest, close to ports and industrial areas (Hatje et al., 2009; Rocha et al., 2016; Andrade et al., 2017), as well as by the finer soil texture, which confers a higher metal accumulation capacity (Harbison, 1986; Soto-Jiménez and Páez-Osuna, 2001; Coringa et al., 2016). Moreover, the more reduced conditions observed in the deep layer, together with the higher content of organic carbon and Fe, may have also promoted the formation of pyrite, a sulfide that is important for metal sequestration in reduced systems (Berner, 1985; Huerta-Díaz and Morse, 1992; Huerta-Díaz et al., 2014).
Cu contents in IM exceeded the background values found for the BTS (16.4±5.6 mg kg−1; Centro de Recursos Ambientais da Bahia, CRA, 2004), as well as the prevention limit (60.0 mg kg−1) established by the Brazilian National Council for the Environment (CONAMA, 2009) (Table 7). Likewise, values exceeded the TEL (18.7 mg kg−1), the concentration below which there is no potential risk of toxic effects to biota, and the effect range-low (ERL) (34.0 mg kg−1) defined by NOAA (1999). Other studies performed in the northern and northeastern areas of the BTS have confirmed Cu enrichment in this region, which has mainly been increasing since the 1970s, with other studies having also shown results above the TEL (Andrade et al., 2017; Santos et al., 2021) and close to the probable effect level (PEL) (108.0 mg kg−1) defined by NOAA (1999), with potential risk to biota, according to the work of Santos et al. (2021).
As for the remaining elements, only Ni showed values above the TEL (15.9 mg kg−1, NOAA, 1999) in IM (WS, 0–5 cm, 16.99±2.62; 15–30 cm, 26.04±1.23), although they were within the prevention limit established by Brazilian legislation (30.0 mg kg−1, CONAMA, 2009) (Table 7) and within the range observed by other studies on the BTS (1.98–17.36 mg kg−1 Otero et al., 2008; 5.09±0.65 mg kg−1, Pereira et al., 2015; <limit of quantification–12.07 mg kg−1, Santos et al., 2021).
Values found for Pb, Zn, and Cr in IM were within the prevention limits set by Brazilian law (CONAMA, 2009), although some samples showed contents that exceeded the background values found in the BTS (CRA, 2004) for Cr and Pb, as well as the TEL for Cr (NOAA, 1999) (Table 7).
In the BTS region, studies have shown Pb enrichment associated with improper waste disposal from a metallurgical plant in the city of Santo Amaro, with soil and sediment pollution in the Subaé estuary (Hatje et al., 2006; Bomfim et al., 2015). A recent study by Gloaguen et al. (2021) found high contents in sediment material from the Subaé River, with high values mainly found in sites close to the plant, with concentrations ranging from 18.3 to 2506 mg kg−1, thus demonstrating active and persistent metal pollution in this region. Likewise, Bomfim et al. (2015) found high values in mangroves in the lower course of the Subaé River, with the highest contents observed in the areas with the greatest river influence and located closest to the plant. Pb values observed in the mangrove forest in PT were lower than those found in previous studies in the Subaé estuary and could be related to the low mobility of this element throughout the river course, with the highest values found closer to the metallurgic plant (Bomfim et al., 2015; Silva et al., 2017; Gloaguen et al., 2021). The observed results could also be related to the low metal retention capacity of soils in PT, based on their predominantly sandy texture and to their lower Fe contents (Harbison, 1986; Soto-Jiménez and Páez-Osuna, 2001; Pittarello et al., 2019).
Distribution of toxic metals among the different reactive fractions showed different patterns, which could impact their bioavailability. Non-residual Cu and Ni were mainly found in the oxidizable fraction (CuRed, NiRed), as has been observed in other study areas (Zhou et al., 2010; Chakraborty et al., 2015; Araújo et al., 2022); this is explained by the affinity of these two metals to organic matter, forming strong complexes in reducing conditions (Zhou et al., 2010; Chakraborty et al., 2016), as well as by the tendency to form sulfides (Morse and Luther, 1999), with high rates of incorporation into pyrite (Huerta-Díaz and Morse, 1992; Morse and Luther, 1999; Otero and Macías, 2003), as shown in Fig. 4. The spatial differences found in terms of both potentially bioavailable contents and total Cu contents (which were higher in IM) suggest increased potential bioavailability with increasing metal enrichment.
Zn and Pb were mainly present in the reactive phase, showing higher mobility than other elements, consistently with observations made by other studies (Coringa et al., 2016; Araújo et al., 2022). These elements are generally found in fraction F1 (exchangeable), associated with carbonates, and adsorbed to Fe and Mn oxides (Espinosa et al., 2011; Coringa et al., 2016; Chen et al., 2022), while values are low in the pyrite fraction (Fig. 4) (Huerta-Díaz and Morse, 1992; Otero and Macías, 2003). Zn and Pb form highly stable sulfides, although they are soluble in acidic environments, suggesting the solubilization of sulfides formed in previous fractions (Morse and Luther, 1999). Conversely, Cr did not show a common distribution pattern among fractions, displaying a variable behavior among the different mangrove sites: it was detected both in the reactive and oxidizable fractions, which could be associated with the variability in oxidation states of this element, which translates into variable partition patterns depending on redox conditions (Otero and Macías, 2003). Moreover, differences in soil chemical parameters, mainly TOC and Fe oxyhydroxide contents, may have influenced the observed distribution patterns. Potentially bioavailable Cr tends to be present in the oxidizable phase in soils, mainly associated with organic matter and without high rates of incorporation into pyrite, with kinetically inert Cr3+ forming sulfides (Morse and Luther, 1999; Otero and Macías, 2003; Zhou et al., 2010). In addition, it may be adsorbed onto Fe and Mn oxyhydroxides or form insoluble Cr hydroxides (Rai et al., 1989).
4.2 Organic Pollutants (PAHs and PCBs)
The values observed for PAHs in soils showed evident spatial variations, except for [B(b+j)F] and [B(a)P], which were higher in PG and CP, in Itaparica Island, than in PT and IM, respectively. The contents found for most PAHs were within the thresholds for environmental quality, except for [ACY] in CP and PT and for [DhA] in CP, which exceeded the TEL (NOAA, 1999). High PAH values in Itaparica Island (CP and PG) have also been found by other studies and associated with oil combustion and activity at marine terminals (Rocha et al., 2012; Almeida et al., 2018; Eça et al., 2021).
PAH ratios in soils (Table 8) were analyzed to determine the sources of hydrocarbons in the studied mangrove sites. The results showed differences among areas, with PAHs in IM mainly associated with petrogenic sources, while the results obtained in the remaining mangroves indicated mixed sources, associated both with a petrogenic origin and with combustion by vehicles.
PCB contents found in soils also showed clear spatial variations, with the highest contents found in IM (16.1 ng g−1) and PG (15.8 ng g−1). Concentrations found in these areas were higher than those observed by Neto et al. (2020) in the BTS (∑PCBs: <limit of quantification–4.7 ng g−1), as well as higher than values measured in Guaratuba bay, a well-preserved estuary in southern Brazil (∑PCBs: <limit of quantification–5.6 ng g−1; Combi et al., 2013), although below the TEL (21.6 ng g−1, NOAA, 1999). The values observed in this study were also lower than those found in the Santos estuary, in São Paulo (∑PCBs: <limit of detection–190.7 ng g−1, Souza et al., 2018), and in Guanabara bay, in Rio de Janeiro (∑PCBs: 17.83–184.16 ng g−1, Souza et al., 2008).
4.3 Toxic Compounds in Ucides cordatus
4.3.1 Trace Elements
The observed results evidenced clear variations in the concentrations of all elements among sites, with different behaviors among metals in terms of their exposure-concentration ratios. Cu and Cr in biota were mainly associated with contents in reactive fractions, with no direct association with total contents in soil, consistently with the observations made by previous studies (Szolnoki and Farsang, 2013; Araújo-Júnior et al., 2016).
The highest Cu values were found during the dry season, possibly related to changes in geochemical conditions and bioavailability because of seasonality (Lessa et al., 2001, 2009; Berrêdo et al., 2016). Lower precipitations lead to lower soil water saturation, promoting air diffusion into the soil and sulfide oxidization (Otero et al., 2010); likewise, reproductive activity of U. cordatus during the dry season promotes soil bioturbation and, consequently, higher levels of aeration of the edaphic system, with a cumulative effect on redox conditions (Araújo-Júnior et al., 2016; Motta et al., 2023). Cr contents in gill tissue, mainly detected in IM and PG, similarly to observations made for Cu, also reflected the contents in the reactive fraction and confirm the higher mobility and bioavailability associated with these geochemical fractions (Espinosa et al., 2011; Coringa et al., 2016).
Unlike for Cu, no association was observed between the concentrations of Zn, Pb, and Ni in crabs and their contents in soils (total concentrations/reactive fraction), consistently with the pattern observed in other studies (Otero et al., 2000; Fakayode and Onianwa, 2002). This suggests that the concentrations of these elements in tissues of U. cordatus result from the complex interaction between exposure and the mechanisms involved in bioaccumulation kinetics associated with metal regulation, in the case of Zn, and with detoxification and elimination mechanisms in the case of elements that do not play any biological role (Rainbow, et al., 2009; Duarte et al., 2019).
Zn and Cu were the elements with the highest concentrations in tissues of U. cordatus, which can be explained by the role played by these elements in the metabolism of crustaceans (Amiard et al., 1987; Rainbow, 2002). These metals showed differences in concentration among tissues, with Zn mainly found in muscle tissue, consistently with observations made by previous studies on the same species in Brazil (Almeida et al., 2014; Bosco-Santos et al., 2017; Silva et al., 2018), while Cu was present in gills and hepatopancreas in those mangroves with the highest accumulation rates (PT and IM), unlike the observations in PG and CP, where contents were mainly present in gill tissue. The results found in this study are consistent with those from previous works, which have also demonstrated a tendency towards high Cu accumulation both in gills and in hepatopancreas in environments highly exposed to metals. Firat et al. (2008), in Iskenderun Bay, an area with an intense agricultural and industrial activity, found high Cu concentrations in gills (827.0 ± 92.6 μg g−1) and hepatopancreas (935.1 ± 50.5 μg g−1) of the crab Charybdis longicolis, showing a distribution pattern and concentrations similar to those found in IM and PT in this study. In low-exposure environments, such as CP and PG, Cu contents were found mainly in gill tissue, similarly to observations in previous studies on U. cordatus in Brazil (Almeida et al., 2014; Ramos and Leite, 2022) and on other crab species in other regions of the world (Portunus pelagicus and Portunus sanguinolentus, Kumar et al., 2019).
Non-essential elements also showed differences in their distribution among tissues, with Pb and Cr mainly present in gills and Ni in hepatopancreas. Unlike Zn and Cu, non-essential elements are not regulated by organisms and are instead accumulated and stored in detoxified forms, associated with metallothioneins, or excreted with the carapace during molting or by other pathways, such as through the gills, guts, or antennal glands (Rainbow, 2002; Marsden and Rainbow, 2004; Rainbow, 2006). The presence of Ni preferentially in hepatopancreas is consistent with the results observed in crabs (Guerardi et al., 2002; Almeida et al., 2014) and is associated with the detoxifying role of this organ, particularly taking into account that this metal lacks any physiological role (non-essential). As in the case of hepatopancreas, the high concentrations found in gills are explained by the higher metabolic activity of this organ, constituting a permeable surface that promotes metal absorption and generally reflecting the concentrations of bioavailable soluble metals in the environment (Rainbow, 2007; Çogun et al., 2017).
Metal contents found in U. cordatus in the studied mangrove forests were different from those observed in other mangroves in Brazil and worldwide. The results for Cu observed in IM and PT were higher than those found in U. cordatus in Brazil to date for all tissues (Table 9) (Harris and Santos, 2000; Almeida et al., 2014; Silva et al., 2018; Ramos and Leite, 2022), while values in CP and PG were close to those found in other regions of Brazil, both in muscle (Almeida et al., 2014; Bosco-Santos et al., 2017; Silva et al., 2018) and in gills (Silva et al., 2018).
Concentrations in hepatopancreas in IM and PT were similar to the values found in the blue crab Callinectes danae in a polluted mangrove forest in São Paulo (Harris and Santos, 2000); likewise, contents in IM were close to those found in crab Charybdis longicolis in a metal-enriched bay in Turkey (Firat et al., 2008). Zn contents in hepatopancreas were higher than those observed in previous studies (Almeida et al., 2014; Ramos and Leite, 2022), unlike contents in muscle and gills, whose values were close to previous data for this species. As for non-essential elements, values of Cr and Pb (in gills) and of Ni (in gills and hepatopancreas) were also higher than previously obtained results for U. cordatus.
Cu contents found in hepatopancreas, a tissue that is also consumed by humans, exceeded the limit set by the World Health Organization (WHO, 1989), which establishes a maximum concentration of 30 μg g−1 (wet weight), corresponding to approximately 150 μg g−1 dry weight of Cu (Annabi et al., 2018). Despite being an essential metal, when Cu is present in excess in organisms, it poses a health risk since it can lead to oxidative damage, which can progress to a series of neurodegenerative diseases, including Alzheimer’s disease and arteriosclerosis (Gaetke and Chow, 2003; Brewer, 2010). Moreover, crustaceans highly exposed to Cu can show ionic and osmoregulatory imbalances, respiratory disorders, oxidative stress, and lethal damage (Bianchini et al., 2004; Grossell et al., 2007; Martins et al., 2011).
4.4 Organic Pollutants (PAHs and PCBs)
Values above the quality limits for PAHs in crabs were found only in CP for ∑[B(a)P, B(a)A, B(b)F, ChR], with [B(a)P] contents below the maximum permissible limit in all sites. Values found for ∑16PAHs in hepatopancreas in this study (82.3–204 ng g −1; mean, 142±35.4 ng g−1) were lower than those observed for U. cordatus in Guanabara bay, in Rio de Janeiro (∑16PAHs = 2290±773 ng g−1; Nudi et al., 2007) and for Callinectes sp. (∑10PAHs=600±66.8 ng g−1) in estuarine systems in Maranhão (Righi et al., 2022), as well as lower than the results found for mollusks in the BTS (∑16PAHs = 459 ng g−1; Eça et al., 2021).
PCB contents also showed spatial differences in U. cordatus tissues, with the highest values in hepatopancreas and muscle found in the mangrove forest in PT (0.60–2.46 ng g−1), showing no clear association with contents in soils, which were highest in IM and PG. Santos et al. (2020) also found higher PCB values in mollusks, both in oysters (Crassotrea rhizophorae) and in mussels (Mytella guyanensis), in the Subaé estuary (9.36–50.1 ng g−1), which could be related to its proximity to the industrial areas located in the northern and northeastern BTS (Hatje et al., 2009; Rocha et al., 2012). Values found in U. cordatus were lower than those recorded by Santos et al. (2020), which is related to the greater capacity of mollusks to bioaccumulate these compounds into their tissues due to their filter-feeding behavior (Goldberg et al., 1978). Likewise, compared with previous studies on crabs, the values observed for U. cordatus were lower than those found in polluted areas in Guanabara bay (Chasmagnathus granulata, ∑PCBs 570.6 ng g−1, Souza et al., 2008) and in China (Scylla serrata, ∑PCBs 32.1–118 ng g−1, Sun et al., 2015), but were close to contents found in Cancer irroratus in Canada (0.12–4.5 ng g−1, Walker et al., 2013).
PCB153 (2,2′,4,4′,5,5′-hexachlorobiphenyl) and PCB138 (2,2′,3,4′,5,5′-hexachlorobiphenyl) were the ones with the highest concentrations in U. cordatus, a common pattern in biological samples (Santos et al., 2020, Sun et al., 2015; Madgett et al., 2022) that can be explained by the high persistence of these congeners due to their chemical structure, with chlorine located in meta/para positions close to the phenyl rings, and to the higher number of chlorine atoms in the molecule. The higher chemical complexity of these compounds makes them more difficult to metabolize and eliminate (Meadows, 1998; Penteado and Vaz, 2001), which slows down their excretion process compared with other compounds with fewer chlorine atoms, such as PCB28 (2,4,4′-trichlorobiphenyl), which showed low values in U. cordatus.
4.5 Genetic and Enzymatic Biomarkers in Ucides cordatus
Many authors (Duarte et al., 2014, 2016; Banci et al., 2017; Santos et al., 2019; Jesus et al., 2021) have revealed biological alterations in U. cordatus in response to exposure to heavy metals present in the environment. These responses include alterations in CAT and GST enzyme activity. In our study, the patterns shown by enzymatic biomarker responses differed among the studied mangrove sites, with higher GST and CAT activities in crabs from Ilha de Maré (IM). The highest GST activity (492 ±17.5 μmol min mg protein−1) was observed in animals collected in IM, contrasting with animals from the RESEX (279 ±59.5 μmol min mg protein−1). While the RESEX is considered to be a conservation area, the GST activity in animals collected at this site was many times higher than values found for U. cordatus individuals collected in areas with heavy metal pollution (Carvalho-Neta et al., 2019; Santos et al., 2019; Jesus et al., 2021). Biochemical biomarker responses in decapod crustaceans may vary according to the species, size, pollutant concentration, and exposure time (Frías-Espericueta et al., 2022). Again according to Frías-Espericueta et al. (2022), the presence of metals in the environment can lead to different effects on enzymatic systems, either increasing or decreasing enzyme activity due to the generation of reactive oxygen species (ROS). Thus, it is difficult to generalize about the results of said responses (Truchet et al., 2023). According to Carvalho-Neta et al. (2019), higher GST activities could be related to heavy metal pollution. Jerome et al. (2017) stated that metals such as Zn ad Cu strongly influence physiological responses in aquatic animals and in their tissues. Therefore, this suggests that crabs from Ilha de Maré could be vulnerable to xenobiotic compounds in this region, a hypothesis that could be confirmed by determining metal concentrations in soils and animals collected at this site.
Catalase activity also varied according to the collection site. The highest enzyme activity was observed in animals collected in Ilha de Maré (47.9 ± 9.7 nmol min mg protein−1), a value that was four times those found in animals from the RESEX (11.5 ± 1.4 nmol min mg protein−1). The results of this study are consistent with findings by Carvalho Neta et al. (2019) and Oliveira et al. (2019), who also reported high catalase activity values in U. cordatus in areas adjacent to port facilities. Jesus et al. (2021) also observed higher CAT activity levels in hepatopancreas of U. cordatus individuals collected in port areas. Catalase, being an antioxidant enzyme, shows increased activity in environments with higher levels of chemical pollution (Jebali et al., 2007; Pereira et al., 2009). Thus, the higher activity of this enzyme observed in animals from IM could be related to the presence of and exposure to xenobiotic compounds. As in the case of GST, CAT values found in animals collected in IM were higher than those observed in U. cordatus from Baía de São Marcos, in northeast Brazil (Jesus et al., 2021) and from the northern coast of Brazil.
Similar to the observations made for enzymatic biomarkers, the highest micronucleated cell frequencies were recorded in crabs from this site (4.0±1.0‰), exceeding the levels observed by Duarte et al. (2016) in Ucides cordatus from well-preserved mangrove forests in São Paulo (Juréia, 0.7±688 1.0‰; Cananéia, 0.4±0.6‰) and similar to values found in crabs from more impacted areas (Cubatão, 5.2±1.9‰; Bertioga, 5.0±1.3‰; Iguape, 3.8±2.5‰).
Duarte et al. (2016) established reference values for micronucleated cell frequencies in U. cordatus from impacted and non-impacted mangrove forests, indicating the conservation status of different mangrove sites in São Paulo state. These authors concluded that uçá crabs in non-impacted areas showed genotoxicity levels lower than 3 micronuclei (MN)‰, while levels in crabs from moderately impacted areas were between 3 and 5 MN‰ and those from highly impacted areas showed frequencies higher than 5 MN‰.
Micronuclei are important markers of the health status of aquatic organisms, and their increase is associated with higher levels of exposure to pollutants (Nudi et al., 2010). Duarte et al. (2017) evaluated the association of heavy metals (such as lead) in uçá crab tissues with genotoxic response, measured in terms of micronucleated blood cells, and observed a significant correlation between both parameters. Therefore, the presence of higher levels of pollutants (mainly of trace elements such as Cu) in IM, together with the pattern observed for enzymes, suggest a greater exposure to pollutants in this mangrove forest, with genotoxicity occurring in resident crabs.
5 Conclusions
Mangrove soils showed spatial heterogeneity in terms of their composition and physicochemical properties, which influenced toxic metal contents and geochemical partition of elements. In this sense, it is worth noting the high levels of metal enrichment in soils of the Ilha de Maré site, particularly of Cu, which showed greater bioavailability during the dry season.
On the other hand, spatial variations in the contents of potentially toxic Cu and Cr observed in crab populations were consistent with spatial variations of metals in soils. Moreover, Cu concentrations observed in U. cordatus exceeded the limits set by the World Health Organization. Cu and Cr concentrations in Ucides cordatus were mainly associated with contents of reactive fractions rather than with total contents in soil, showing an increase in tissue accumulation in response to increased bioavailability. Meanwhile, for Zn, Pb, and Ni, no clear relationships were established between contents in soil (both total concentrations and contents in reactive fractions) and in tissues, suggesting that concentrations of these elements in tissues not only reflect exposure, but also result from the interaction of factors related both to environmental concentrations and to bioaccumulation kinetics (metal accumulation, distribution among tissues, regulation, and detoxification).
The studied mangrove sites showed PAH contents in soils and in crabs that were below the thresholds for environmental quality, except for [ACY] in CP and PT and [DhA] in CP, which exceeded the TEL. Likewise, values above the quality limit were observed in muscle tissue in CP, suggesting that this region of the BTS is enriched in these compounds, posing potential risks to human health.
Crabs from Ilha de Mare showed a different pattern in their biomarker responses, with evidence of oxidative stress and genotoxicity, results that show sublethal damages in populations of U. cordatus as a response to enrichment of pollutants in this mangrove, mainly of trace elements. The high contents detected in biota pose a risk not only to crab populations, but also for humans, taking into account the consumption of these crustaceans and the consequent transfer of these elements via the trophic chain. This warrants the need to assess the consumption of these crustaceans and the ecotoxicological risks in this area.
Data Availability
The data with which this article has been prepared are part of the doctoral thesis of Paulina Guevara (Space-temporal variability of the concentration of trace metals in soils and sediments in the Ria de Ortigueira in relation to the sources and residual dynamics of marine currents) and they can be accessed in the MINERVA repository of the University of Santiago de Compostela (https://minerva.usc.es/xmlui/?locale-attribute=gl). The data can also be available from the author (Xose L Otero: xl.otero@usc.es).
References
Aebi, H. E. (1987). Catalase. In J. Bergmeyer & M. Grossl (Eds.), Methods of Enzymatic Analysis (Vol. 3, 3rd ed., pp. 273–286). VCH.
Almeida, E. V., Kütter, V. T., & Silva-Filho, E. V. (2014). Metais traço em caranguejos de mangue Ucides cordatus (L., 1763) do leste da baía de Guanabara (Sudeste do Brasil). In J. E. Marcovecchio, S. E. Botté, & R. H. Freije (Eds.), Procesos Geoquímicos Superficiales en Iberoamérica (pp. 243–260).
Almeida, M., Nascimento, D. V., Mafalda-Júnior, P. O., Patire, V. F., & Albergaria-Barbosa, A. C. R. (2018). Distribution and sources of polycyclic aromatic hydrocarbons (PAHs) in surface sediments of a Tropical Bay influenced by anthropogenic activities (Todos os Santos Bay, BA, Brazil). Marine Pollution Bulletin, 137, 399–407.
Amiard, J. C., Amiard-Triquet, C., Berthet, B., & Metayer, C. (1987). Comparative study of the patterns of bioaccumulation of essential (Cu, Zn) and non-essential (Cd, Pb) trace metals in various estuarine and coastal organisms. Journal of Experimental Marine Biology and Ecology, 106, 73–89.
Annabi, A., Bardelli, R., Vizzini, S., & Mancinelli, G. (2018). Baseline assessment of heavy metals content and trophic position of the invasive blue swimming crab Portunus segnis (Forskal, 1775) in the Gulf of Gabes (Tunisia). Marine Pollution Bulletin, 136, 454–463.
Andrade, R. L. B., Hatje, V., Masqué, P., Zurbrick, C. M., Boyle, E. A., & Santos, W. P. C. (2017). Chronology of anthropogenic impacts reconstructed from sediment records of trace metals and Pb isotopes in Todos os Santos Bay (NE Brazil). Marine Pollution Bulletin, 125, 459–471.
Araújo, P. R. M., Biondi, C. M., Nascimento, C. W. A., Silva, F. B. V., Ferreira, T. O., & Alcântara, S. F. (2022). Geospatial modeling and ecological and human health risk assessments of heavy metals in contaminated mangrove soils. Marine Pollution Bulletin, 177, 113489.
Araújo Júnior, J. M. C., Ferreira, T. O., Suarez-Abelenda, M., Nóbrega, G. N., Albuquerque, A. G. B. M., Bezerra, A. C., & Otero, X. L. (2016). The role of bioturbation by Ucides cordatus crab in the fractionation and bioavailability of trace metals in tropical semiarid mangroves. Marine Pollution Bulletin, 111, 194–202.
Banci, K. R. S., Mori, G. M., Oliveira, M. A., Paganelli, F. L., Pereira, M. R., & Pinheiro, M. A. A. (2017). Can environmental pollution by metals change genetic diversity? Ucides cordatus (Linnaeus, 1763) as a study case in Southeastern Brazilian mangroves. Marine Pollution Bulletin, 116, 440–447.
Berner, R. A. (1984). Sedimentary pyrite formation: An update. Geochimica et Cosmochimica Acta, 48, 605–615. https://doi.org/10.1016/0016-7037(84)90089-9
Berner, R. A., Leeuw, J. W., Spiro, B., Murchison, D. G., & Eglinton, G. (1985). Sulphate reduction, organic matter decomposition and pyrite formation. Philosophical Transactions of the Royal Society of London. Series A, Mathematical and Physical Sciences, 315, 25–38.
Berrêdo, J. F., Costa, M. L., Vilhena, M. S. P., & Matos, C. R. L. (2016). Modificações nas propriedades físico-químicas de sedimentos de manguezais submetidos ao clima amazônico. Boletim do Museu Paraense Emílio Goeldi-Ciências Naturais, 11(3), 313–328.
Bianchini, A., Martins, S. E. G., & Barcarolli, I. F. (2004). Mechanism of acute copper toxicity in euryhaline crustaceans: Implications for the Biotic Ligand Model. International Congress Series, 1275, 189–194. https://doi.org/10.1016/j.ics.2004.08.074
Bomfim, M. R., Santos, J. A. G., Costa, O. V., Otero, X. L., Boas, G. S. V., Capelão, V. S., Santos, E. S., & Nacif, P. G. S. (2015). Genesis, characterization, and classification of mangrove soils in the Subaé River Basin, Bahia, Brazil. Revista Brasileira de Ciência do Solo, 39, 1247–1260.
Bosco-Santos, A., Silva, W. L., Silva-Filho, E. V., Souza, M. D. C., Dantas, E. L., & Navarro, M. S. (2017). Fractionation of rare earth and other trace elements in crabs, Ucides cordatus, from a subtropical mangrove affected by fertilizer industry. Journal of Environmental Sciences, 54, 69–76.
Brewer, G. J. (2010). Risks of copper and iron toxicity during aging in humans. Chemical Research in Toxicology, 23, 319–326.
Carvalho-Neta, R. N. F., Andrade, T. S. O. M., Oliveira, S. R. S., Torres-Júnior, A. R., Cardoso, W. S., Santos, D. M. S., Batista, W. S., Serra, I. M. R. S., & Brito, N. M. (2019). Biochemical and morphological responses in Ucides cordatus (Crustacea, Decapoda) as indicators of contamination status in mangroves and port areas from northern Brazil. Environmental Science and Pollution Research, 26, 15884–15893. https://doi.org/10.1007/s11356-019-04849-0
Carvalho, I. G. S. (2020). Actividad Impedida de Recolectores de Mariscos y Pescadores Artesanales de la Isla de Maré, Baía de Todos os Santos. Revista de Ciências Jurídicas, 21(1), 10–16.
Carvalho, I. G. S., & Vidal, J. P. (2020). La comunidad tradicional quilombola de pescadores artesanales y recolectoras de mariscos de la Isla de Maré, en Brasil, y su exclusión social, histórica y cultural: un proceso de invisibilidad. Revista Interdisciplinar em Educação e Territorialidade–RIET, 1(1), 48–70.
Celino, J. J., & Queiroz, A. F. S. (2006). Fonte e grau da contaminação por hidrocarbonetos policíclicos aromáticos (HPAs) de baixa massa molecular em sedimentos da baía de Todos os Santos, Bahia. Rem: Revista Escola de Minas, 59(3), 265–270.
Chakraborty, P., Ramteke, D., & Chakraborty, S. (2015). Geochemical partitioning of Cu and Ni in mangrove sediments: Relationships with their bioavailability. Marine Pollution Bulletin, 93(1), 194–201.
Chakraborty, P., Chakraborty, S., Vudamala, K., Sarkar, A., & Nath., R. N. (2016). Partitioning of metals in different binding phases of tropical estuarine sediments: Importance of metal chemistry. Environmental Science and Pollution Research, 23, 3450–3462.
Chen, B., He, R., Cai, P., Huang, G., & Wang, F. (2022). Geochemical speciation, risk assessment, and sources identification of heavy metals in mangrove surface sediments from the Nanliu River Estuary of the Beibu Gulf, China. Sustainability, 14, 9112.
Combi, T., Tanihuchi, S., Figueira, R. C. L., Mahiques, M. M., & Martins, C. C. (2013). Spatial distribution and historical input of polychlorinated biphenyls (PCBs) and organochlorine pesticides (OCPs) in sediments from a subtropical estuary (Guaratuba Bay, SW Atlantic). Marine Pollution Bulletin, 70, 247–252.
Conselho Nacional do Meio Ambiente – CONAMA. (2009). Dispõe sobre critérios e valores orientadores de qualidade do solo quanto à presença de substâncias químicas e estabelece diretrizes para o gerenciamento ambiental de áreas contaminadas por essas substâncias em decorrência de atividades antrópicas. Brasília, 2009, 20 BRASIL. Resolução n° 420, de 28 de dezembro de.
Çoğun, H. Y., Firat, O., Aytekin, T., Firidin, G., Firat, Ö., Varkal, H., Temiz, O., & Kargin, F. (2017). Heavy Metals in the Blue Crab (Callinectes sapidus) in Mersin Bay, Turkey. Bulletin of Environmental Contamination and Toxicology, 98, 824–829.
Coringa, J. E. S., Pezza, L., Coringa, E. A. O., & Weber, O. L. S. (2016). Distribuição geoquímica e biodisponibilidade de metais traço em sedimentos no Rio Bento Gomes, Poconé - MT, Brasil. Acta Amazonica, 46, 161–174.
Countryman, P. I., & Heddle, J. A. (1976). The production of micronuclei from chromosome aberrations in irradiated cultures of human lymphocytes. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis, 41(2-3), 321–332. https://doi.org/10.1016/0027-5107(76)90105-6
Centro de Recursos Ambientais – CRA. (2004). Diagnóstico da concentração de metais pesados e hidrocarbonetos de petróleo nos sedimentos e biota da Baía de Todos os Santos. Consórcio BTS Hydros CH2MHILL. .
Duarte, L. F. A., Duran, R. S., Mendonça, J. T., & Pinheiro, M. A. A. (2014). Fishery of the uçá crab Ucides cordatus (Linnaeus, 1763) in a mangrove area in Cananéia, State of São Paulo, Brazil: Fishery performance, exploitation patterns and factors affecting the catches. Brazilian Journal of Oceanography, 62, 187–199.
Duarte, L. F. A., Souza, C. A., Nobre, C. R., Pereira, C. D. S., & Pinheiro, M. A. A. (2016). Multi-level biological responses in Ucides cordatus (Linnaeus, 1763) (Brachyura,Ucididae) as indicators of conservation status in mangrove áreas from the western atlantic. Ecotoxicology and Environmental Safety, 133, 176–187.
Duarte, L. F. D., Souza, C. A., Pereira, C. D. S., & Pinheiro, M. A. A. (2017). Metal toxicity assessment by sentinel species of mangroves: In situ case study integrating chemical and biomarkers analyses. Ecotoxicology and Environmental Safety, 145, 367–376.
Duarte, L. F. A., Moreno, J. B., Catharino, M. G. M., Moreira, E. G., Trombini, C., & Pereira, C. D. S. (2019). Mangrove metal pollution induces biological tolerance to Cd on a crab sentinel species subpopulation. Science of the Total Environment, 687, 768–779.
Eça, G. F., Albergaria-Barbosa, A. C. R., Souza, M. M., Costa, P. G., Leite, A. S., Fillmann, G., & Hatje, V. (2021). Polycyclic aromatic hydrocarbons in sediments and shellfish from Todos os Santos bay, Brazil. Marine Pollution Bulletin, 173, 112944.
Espinosa, L. F., Parra, J. P., & Villamil, C. (2011). Determinación del contenido de metales pesados en las fracciones geoquímicas del sedimento superficial asociado a los manglares de la Ciénaga Grande de Santa Marta, Colombia. Boletín de Investigaciones Marinas y Costeras-INVEMAR, 40(1), 7–23.
Fakayode, S. O., & Onianwa, P. C. (2002). Heavy metal contamination of soil, and bioaccumulation in Guinea grass (Panicum maximum) around Ikeja Industrial Estate, Lagos, Nigeria. Environmental Geology, 43, 145–150.
Firat, Ö., Gök, G., Çoğun, H. Y., Yüzereroğlu, T. A., & Kargin, F. (2008). Concentrations of Cr, Cd, Cu, Zn and Fe in crab Charybdis longicollis and shrimp Penaeus semisulcatus from the Iskenderun Bay, Turkey. Environmental Monitoring Assessment, 147, 117–123.
Frías-Espericueta, M. G., Bautista-Covarrubias, J. C., Osuna-Martínez, C. C., Delgado-Alvarez, C., Bojórquez, C., Aguilar-Juárez, M., Roos-Muñoz, S., Osuna-López, I., & Páez-Osuna, F. (2022). Metals and oxidative stress in aquatic decapod crustaceans: A review with special reference to shrimp and crabs. Aquatic Toxicology, 242. https://doi.org/10.1016/j.aquatox.2021.106024
Gaetke, L. M., & Chow, C. K. (2003). Copper toxicity, oxidative stress, and antioxidant nutrientes. Toxicology, 189, 147–163.
Gloaguen, T. V., Motta, P. N. S. D., & Couto, C. F. (2021). A grain-size correction for metal pollution indexes in river sediments. International Journal of Sediment Research, 36, 362–372.
Goldberg, E. D., Bowen, V. T., Farrington, J. W., Harvey, G., Martin, J. H., Parker, P. L., Risebrough, W., Schneider, E., & Gamble, E. (1978). The mussel watch. Environmental Conservation, 5, 1–25.
Grossel, M., Blanchard, J., Brix, K. V., & Gerdes, R. (2007). Physiology is pivotal for interactions between salinity and acute copper toxicity to fish and invertebrates. Aquatic Toxicology, 84, 162–172. https://doi.org/10.1016/j.aquatox.2007.03.026
Guerardi, F., Barbaresi, S., Vaselli, O., & Bencini, A. (2002). A comparison of trace metal accumulation in indigenous and alien freshwater macro-decapods. Marine and Freshwater Behaviour and Physiology, 35(3), 179–188.
Harbison, P. (1986). Mangrove muds-A sink and a source for trace metals. Marine Pollution Bulletin, 17(6), 246–250.
Harris, R. R., & Santos, M. C. F. (2000). Heavy metal contamination and physiological variability in the Brazilian mangrove crabs Ucides cordatus and Callinectes danae (Crustacea: Decapoda). Marine Biology, 137, 691–703.
Hatje, V., Barros, F., Figueiredo, D. G., Santos, V. L. C. S., & Peso-Aguiar, M. C. (2006). Trace metal contamination and benthic assemblages in Subaé estuarine system, Brazil. Marine Pollution Bulletin, 52, 969–987.
Hatje, V.; Bícego, M. C.; Carvalho, G. C.; Andrade, J. B. (2009). Contaminação química. In: Hatje, V. Andrade, J. B. (Org.). Baia de Todos os Santos: aspectos oceanográficos. : EDUFBA, 306.
Huerta-Díaz, M. A., & Morse, J. W. (1992). Pyritization of trace metals in anoxic marine sediments. Geochimica et Cosmochimica, 56, 2681–2702.
Huerta-Diaz, M. A., Barbosa, A. M., Otero, X. L., Valdivieso-Ojeda, J., & Amaro-Franco, E. C. (2014). High variability in geochemical partitioning of iron, manganese and harmful trace metals in sediments of the mining port of Santa Rosalia, Baja California Sur, Mexico. Journal of Geochemical Exploration, 145, 51–63.
Jebali, J., Banni, M., Almeida, E. A., & Boussetta, H. (2007). Oxidative DNA damage levels and catalase activity in the clam Ruditapes decussatus as pollution biomarkers of Tunisian marine environment. Environmental Monitoring and Assessment, 124, 195–200.
Jerome, F. C., Hassan, A., Omoniyi-Esan, G. O., Odujoko, O. O., & Chukwuka, A. V. (2017). Metal uptake, oxidative stress and histopathological alterations in gills and hepatopancreas of Callinectes amnicola exposed to industrial efluente. Ecotoxicology and Environmental Safety, 139, 179–193. https://doi.org/10.1016/j.ecoenv.2017.01.032
Jesus, W. B., Andrade, T. S. O. M., Soares, S. H., Pinheiro-Souza, D. B., Oliveira, S. R. S., Torres, H. S., Protazio, G. S., Silva, D. S., Santos, D. M. S., Carvalho-Neta, A. V., Benjamin, L. A., & Carvalho-Neta, R. N. F. (2021). Biomarkers and occurrences of heavy metals in sediment and the bioaccumulation of metals in crabs (Ucides cordatus) in impacted mangroves on the Amazon coast, Brazil. Chemosphere, 271, 129444. https://doi.org/10.1016/j.chemosphere.2020.129444
Joksimović, D., & Stanković, S. (2012). Accumulation of trace metals in marine organisms of the southeastern Adriatic coast, Montenegro. Ecotoxicology and Environmental Safety, 77(1), 105–117.
Keen, J. H., Habig, W. H., & Jakoby, W. B. (1976). Mechanism for the several activities of the glutathione S-transferases. Journal of Biological Chemistry, 251(20), 6183–6168.
Kroon, F., Streten, C., & Harries, S. (2017). A protocol for identifying suitable biomarkers to assess fish health: A systematic review. PLoS One. https://doi.org/10.1371/journal.pone.0174762
Kumar, S. B., Padhi, R. K., & Satpathy, K. K. (2019). Trace metal distribution in crab organs and human health risk assessment on consumption of crabs collected from coastal water of South East coast of India. Marine Pollution Bulletin, 141, 273–282.
Lam, P. K. (2009). Use of biomarkers in environmental monitoring. Ocean & Coastal Management, 5(2), 348–354.
Lessa, G. C., Dominguez, J. M. L., Bittencourt, A. C. S. P., & Brichta, A. (2001). The Tides and Tidal Circulation of Todos os Santos Bay, Northeast Brazil: A general characterization. Anais da Academia Brasileira de Ciências, 73(2), 245–261.
Lessa, G. C., Cirano, M., Genz, F., Tanajura, C. A. S., & Silva, R. R. (2009). Oceanografia física. In In: Hatje, V. Andrade, J. B. (Org.). (Ed.), Baia de Todos os Santos: aspectos oceanográficos. EDUFBA.
Madgett, A. S., Yates, K., Webster, L., McKenzie, C., Brownlow, A., & Moffat, C. F. (2022). The concentration and biomagnification of PCBs and PBDEs across four trophic levels in a marine food web. Environmental Pollution, 309, 119752.
Marsden, I. D., & Rainbow, P. S. (2004). Does the accumulation of trace metals in crustaceans affect their ecology—The amphipod example? Journal of Experimental Marine Biology and Ecology, 300, 373–408.
Martins, C. M. G., Barcarolli, I. F., Menezes, E. J., Giacomin, M. M., Wood, C. M., & Bianchini, A. (2011). Acute toxicity, accumulation and tissue distribution of copper in the blue crab Callinectes sapidus acclimated to different salinities: In vivo and in vitro studies. Aquatic Toxicology, 101, 88–99.
McLeod, E., & Salm, R. V. (2006). Managing mangroves for resilience to climate change (p. 64). IUCN.
Meadows, J. C., Echols, K. R., Huckins, J. N., Borsuk, F. A., Carline, R. F., & Tillitt, D. E. (1998). Estimation of uptake rate constants for PCB congeners accumulated by semipermeable membrane devices and brown trout (Salmo trutta). Environmental Science & Technology, 32, 1847–1852.
Morse, J. W., & Luther, G. W. (1999). Chemical influences on trace metal-sulfide interactions in anoxic sediments. Geochimica et Cosmochimica Acta, 63, 3373–3378.
Mota, T. A., Pinheiro, M. A. A., Evangelista-Barreto, N. S., & Rocha, S. S. (2023). Density and extractive potential of “uçá”-crab, Ucides cordatus (Linnaeus, 1763), in mangroves of the “Todos os Santos” Bay, Bahia, Brazil. Fisheries Research, 265, 106733.
Neto, B. M. T. S., Combi, T., Taniguchi, S., Albergaria-Barbosa, A. C. R., Ramos, R. B., Figueira, R. C. L., & Montone, R. C. (2020). Persistent organic pollutants (POPs) and personal care products (PCPs) in the surface sediments of a large tropical bay (Todos os Santos Bay, Brazil). Marine Pollution Bulletin, 161, 111818.
National Oceanic and Atmospheric Administration - NOAA. (1999). Screening Quick Reference Tables (p. 12p). National Oceanic and Atmospheric Administration.
Nordhaus, I., Wolff, M., & Diele, K. (2006). Litter processing and population food intake of the mangrove crab Ucides cordatus in a high intertidal forest in northern Brazil. Estuarine. Coastal and Shelf Science, 67, 239–250.
Nordhaus, I., Diele, K., & Wolff, M. (2009). Activity patterns, feeding and burrowing behaviour of the crab Ucides cordatus (Ucididae) in a high intertidal mangrove forest in North Brazil. Journal of Experimental Marine Biology and Ecology, 374, 104–112.
Nudi, A. H., Wagener, A. L. R., Francioni, E., Scofield, A. L., Sette, C. B., & Veiga, A. (2007). Validation of Ucides cordatus as a bioindicator of oil contamination and bioavailability in mangroves by evaluating sediment and crab PAH records. Environment International, 33, 315–327.
Nudi, A. H., Wagener, A. L. R., Francioni, E., Sette, C. B., Sartori, A. V., & Scofield, A. L. (2010). Biomarkers of PAHs exposure in crabs Ucides cordatus: Laboratory assay and field study. Environmental Research, 110, 137–145.
Oliveira, S. R. S., Batista, W. S., Souza, J. B. M., Noleto, K. S., Lima, I. M. A., Andrade, T. S. O. M., Cardoso, W. S., & Carvalho-Neta, R. N. F. (2019). Enzymatic and histological biomarkers in Ucides cordatus (Crustacea, Decapoda) in an industrial port on the North Coast of Brazil. Bulletin of Environmental Contamination and Toxicology, 102, 802–810. https://doi.org/10.1007/s00128-019-02594-1
Oliveira, S. R. S., Oliveira, L. B., Moreno, L. C. G. A., & Carvalho-Neta, R. N. F. (2024). Biomarker responses in Ucides Cordatus (Crusacea; Decapoda) to assess the degree of exposure to aquatic pollution of an estuarine and port region of the Brazilian Amazon. https://doi.org/10.2139/ssrn.4699471
Otero, X. L., Sánchez, J. M., & Macías, F. (2000). Bioaccumulation of heavy metals in thionic fluvisols by a marine polychaete: The role of metal sulfides. Journal of Environmental Quality, 29, 1133–1141.
Otero, X. L., & Macías, F. (2003). Spatial variation in pyritization of trace metals in salt-marsh soils. Biogeochemistry, 62, 59–86.
Otero, O. M. F., Barbosa, R. M., Queiroz, A. F. S., Castro, A. M., Macêdo, B. L. F. (2008). Valores de referência para metais traço nos sedimentos de manguezais da Baía de Todos os Santos. In: Queiroz, A.F. S. & Celino, J.J. (Coords.), Avaliação de ambientes na Baía de Todos os Santos: aspectos geoquímicos, geofísicos e biológicos. : UFBA, p. 59-72.
Otero, X. L., Ferreira, T. O., Huerta-Díaz, M. A., Partiti, C. S. M., Souza, V., Vidal- Torrado, P., & Macías, F. (2009). Geochemistry of iron and manganese in soils and sediments of a mangrove system, Island of Pai Matos (Cananeia -SP, Brazil). Geoderma, 148(3–4), 318–335.
Otero, X. L., Souza, M. L., & Macías, F. (2010). Iron and trace metal geochemistry in mangrove soils. In X. L. O. Pérez & F. M. Vázquez (Eds.), Biogeochemistry and Pedogenetic Process in Saltmarsh and Mangrove Systems (pp. 1–24). Nova Science Publishers Inc..
Penteado, J. C. P., & Vaz, J. M. (2001). O legado das Bifenilas Policloradas (PCB´s). Química Nova, 24(3), 390–398.
Pereira, P., Pablo, H., Subida, M. D., Vale, C., & Pachego, M. (2009). Biochemical responses of the shore crab (Carcinus maenas) in a eutrophic and metal-contaminated coastal system (Óbidos lagoon,Portugal). Ecotoxicology and Environmental Safety, 72, 1471–1480.
Pereira, T. S., Moreira, I. T. A., Oliveira, O. M. C., Rios, M. C., Filho, W. A. C. S., Almeida, M., & Carvalho, G. C. (2015). Distribution and ecotoxicology of bioavailable metals and As in surface sediments of Paraguaçu estuary, Todos os Santos Bay, Brazil. Marine Pollution Bulletin, 99, 166–177.
Pinheiro, M. A. A., Silva, P. P. G., Duarte, L. F. A., Almeida, A. A., & Zanotto, F. P. (2012). Accumulation of six metals in the mangrove crab Ucides cordatus (Crustacea: ucididae) and its food source, the red mangrove Rhizophora mangle (Angiosperma: Rhizophoraceae). Ecotoxicology and Environmental Safety, 81, 114–121.
Pinheiro, M. A. A., Duarte, L. F. A., Toledo, T. R., Adam, M. L., & Torres, R. A. (2013). Habitat monitoring and genotoxicity in Ucides cordatus (Crustacea: Ucididae), as tools to manage a mangrove reserve in southeastern Brazil. Environmental Monitoring and Assessment, 185, 8273–8285.
Pinheiro, M.A.A., Santos, L.C.M., Souza, C.A., João, M.C.A., Neto, J.D., Ivo, C.T.C. (2016). Avaliação do caranguejo-uçá (Ucides cordatus) (Linnaeus, 1763) (Decapoda: Ucididae), in: Pinheiro, M.A.A., Boos, H. (Eds.) Livro vermelho dos crustáceos do Brasil. Avaliação 2010–2014. Sociedade Brasileira de Carcinologia, 441–456.
Pittarello, M., Busato, J. G., Carletti, P., Sodré, F. F., & Dobbss, L. B. (2019). Dissolved humic substances supplied as potential enhancers of Cu, Cd, and Pb adsorption by two diferente mangrove sediments. Journal of Soils and Sediments, 19, 1554–1565.
Rai, D., Eary, L. E., & Zachara, J. M. (1989). Environmental chemistry of chromium. Science of the Total Environment, 86, 15–23.
Rainbow, P. S. (2002). Trace metal concentrations in aquatic invertebrates: Why and so what? Environmental Pollution, 120, 497–507.
Rainbow, P. S. (2006). Biomonitoring of trace metals in estuarine and marine environments. Australasian Journal of Ecotoxicology, 12, 107–122.
Rainbow, P. S. (2007). Trace metal bioaccumulation: Models, metabolic availability and toxicity. Environment International, 33, 576–582.
Rainbow, P. S., Smith, B. D., & Luoma, S. N. (2009). Biodynamic modelling and the prediction of Ag, Cd and Zn accumulation from solution and sediment by the polychaete Nereis diversicolor. Marine Ecology Progress Series, 390, 145–155.
Ramos, R. J., & Leite, G. R. (2022). Disposition of trace elements in the mangrove ecosystem and their effects on Ucides cordatus (Linnaeus, 1763) (Crustacea, Decapoda). Biometals, 35, 853–873.
Rao, M. N., Anjaneyulu, A., Parthipan, V. D., Ram, A., Pradhan, U. K., Krishnan, U., & Siddaiha, V. (2023). Biomarker responses in the Coilia dussumieri exposed to petroleum hydrocarbons contamination in urbanized estuaries along the west coast of India. Environmental Geochemistry and Health, 45, 7727–7740.
Rauret, G., López-Sánchez, J. F., Sahuquillo, A., Rubio, R., Davidson, C., Ureb, A., & Quevauvillerc, P. H. (1998). Improvement of the BCR three step sequential extraction procedure prior to the certification of new sediment and soil reference materials. Journal of Environmental Monitoring, 1, 57–61.
Reimann, C., Filzmoser, P., Garrett, R. G., & Dutter, R. (2008). Statistical Data Analysis Explained, Statistical Data Analysis Explained: Applied Environmental Statistics with R. John Wiley & Sons, Ltd.
Righi, B. D. P., Abujamara, L. D., Barcarolli, I. F., Jorge, M. B., Zebral, Y. D., Costa, P. G., Martinez, C. B. R., & Bianchini, A. (2022). Response of biomarkers to metals, hydrocarbons and organochlorine pesticides contamination in crabs (Callinectes ornatus and C. bocourti) from two tropical estuaries (São José and São Marcos bays) of the Maranhão State (northeastern Brazil). Chemosphere, 288, 132649.
Rocha, G. O., Guarieiro, A. L. N., Andrade, J. B., Eça, G. F., Aragão, N. M., Aguiar, R. M., Korn, M. G. A., Brito, G. B., Moura, C. W. N., & Hatje, V. (2012). Contaminação na Baía de Todos os Santos. Revista Virtual de Química, 5, 583–610.
Rocha, T. S., Sales, E. A., Beretta, M., & Oliveira, I. B. (2016). Effects of dredging at Aratu port in All Saints Bay, Brazil: Monitoring the metal content in water and sediments. Environmental Monitoring and Assessment, 188, 394. https://doi.org/10.1007/s10661-016-5396-y
Santos, C. C. M., Costa, J. F. M., Santos, C. R. M., & Amado, L. L. (2019). Influence of seasonality on the natural modulation of oxidative stress biomarkers in mangrove crab Ucides cordatus (Brachyura, Ucididae). Comparative Biochemistry and Physiology, Part A, 227, 146–153.
Santos, L. L., Miranda, D., Hatje, V., Alberfaria-Barbosa, A. C. R., & Leonel, J. (2020). PCBs occurrence in marine bivalves and fish from Todos os Santos Bay, Bahia, Brazil. Marine Pollution Bulletin, 154, 111070.
Santos, M. V. S., Silva Júnior, J. B., Melo, V. M. M., Souza, D. S., Hadlich, G. M., & Oliveira, O. M. C. (2021). Evaluation of metal contamination in mangrove ecosystems near oil refining areas using chemometric tools and geochemical indexes. Marine Pollution Bulletin, 166, 112179.
Schumacher, B. A. (2002). Methods for the determination of total organic carbon (TOC) in soils and sediments. Ecological Risk Assessment Support Center, U.S. EPA.
Silva, G. S., Gloaguen, T. V., Couto, C. F., & Motta, P. N. S. D. (2017). Persistence and mobility of metals in an estuarine environment 25 years after closure of a lead smelter, Bahia State, Brazil. Environmental Earth Sciences, 76, 548.
Silva, B. M. S., Morales, G. P., Gutjahr, A. L. N., Faial, K. C. F., & Carneiro, B. S. (2018). Bioacumulation of trace elements in the crab Ucides cordatus (Linnaeus, 1763) from the macrotidal mangrove coast region of the Brazilian Amazon. Environmental Monitoring and Assessment, 190, 214. https://doi.org/10.1007/s10661-018-6570-1
Soares, L. S. H., Salles, A. C. R., Lopez, J. P., Muto, E., Y., Giannini, R. (2009). Pesca e produção pesqueira. In: Hatje, V. Andrade, J. B. (Org.). Baia de Todos os Santos: aspectos oceanográficos. : EDUFBA.
Soto-Jimézes, M. F., & Páez-Osuna, F. (2001). Distribution and normalization of heavy metal concentrations in mangrove and lagoonal sediments from Mazatlán Harbor (SE Gulf of California). Estuarine, Coastal and Shelf Science, 53, 259–274.
Souto, F. J. B. (2008). Bosque de mangues e a pesca artesanal no Distrito de Acupe (Santo Amaro, Bahia): uma abordagem etnoecológica. Acta Scientiarum. Biological Sciences, 30(3), 275–282.
Souza, A. S., Torres, J. P. M., Meire, R. O., Neves, R. C., Couri, M. S., & Serejo, C. S. (2008). Organochlorine pesticides (OCs) and polychlorinated biphenyls (PCBs) in sediments and crabs (Chasmagnathus granulata, Dana, 1851) from mangroves of Guanabara Bay, Rio de Janeiro State, Brazil. Chemosphere, 73, 186–192.
Souza, A. C., Taniguchi, S., Figueira, R. C. L., Montone, R. C., Bícego, M. C., & Martins, C. C. (2018). Historical records and spatial distribution of high hazard PCBs levels in sediments around a large South American industrial coastal area (Santos Estuary, Brazil). Journal of Hazardous Materials, 360, 428–435.
Sun, Y.-X., Zhang, Z.-W., Xu, X.-R., Hu, Y.-X., Luo, X.-J., Cai, M.-G., & Mai, B.-X. (2015). Bioaccumulation and biomagnification of halogenated organic pollutants in mangrove biota from the Pearl River Estuary, South China. Marine Pollution Bulletin, 99, 150–156.
Szolnoki, Z., & Farsang, A. (2013). Evaluation of metal mobility and bioaccessibility in soils of urban vegetable gardens using sequential extraction. Water, Air, & Soil Pollution, 224, 1737. https://doi.org/10.1007/s11270-013-1737-4
Truchet, D. M., Negro, C. L., Buzzi, N. S., Mora, M. C., & Marcovecchio, J. E. (2023). Assessment of metal contamination in an urbanized estuary (Atlantic Ocean) using crabs as biomonitors: A multiple biomarker approach. Chemosphere, 312(Part 1), 137317.
Van der Oost, R., Beyer, J., & Vermeulen, N. P. (2003). Fish bioaccumulation and biomarkers in environmental risk assessment: A review. Environ Toxicol Pharmacol, 13(2), 57–149.
Walker, A. N., Golden, R., & Horst, M. N. (2010). Morphologic effects of in vivo acute exposure to the pesticide methoprene on the hepatopancreas of a non-target organismo Homarus americanus. Ecotoxicology and Environmental Safety, 73, 1867–1874.
Walker, T. R., MacAskill, D., & Weaver, P. (2013). Legacy contaminant bioaccumulation in rock crabs in Sydney Harbour during remediation of the Sydney Tar Ponds, Nova Scotia, Canada. Marine Pollution Bulletin, 77, 412–417.
World Health Organization – WHO. (1989). Heavy Metals-Environmental Aspects. Environment Health Criteria.
Yamamoto, F. Y., Onishi, K., Ralha, T. R., Silva, L. F. O., Deda, B., Pessali, T. Y. C., Souza, C., Ribeiro, C. A. O., & Abessa, D. M. S. (2023). Earlier biomarkers in fish evidencing stress responses to metal and organic pollution along the Doce River Basin. Environmental Pollution, 329. https://doi.org/10.1016/j.envpol.2023.121720
Yunker, M. B., Macdonald, R. W., Vingarzan, R., Mitchell, R. H., Goyette, D., & Sylvestre, S. (2002). PAHs in the Fraser River basin: A critical appraisal of PAH ratios as indicators of PAH source and composition. Organic Geochemistry, 33, 489–515.
Zhou, Y., Zhao, B., Peng, Y., & Chen, G. (2010). Influence of mangrove reforestation on heavy metal accumulation and speciation in intertidal sediments. Marine Pollution Bulletin, 60, 1319–1324.
Acknowledgements
We would like to thank María José Santiso and Francisco Casás for their assistance with laboratory work.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This research was co-funded by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) do Ministério da Ciência, Tecnologias e Inovações do Brasil, Project titled “Desenvolvimento do Índice de Qualidade das Florestas de manguezais na Baía de Todos Santos (BTS), Bahia” (no. 441389/2017-1), and the Consellería de Educación, Universidade e Formación Profesional-Xunta de Galicia (Axudas á consolidación e estruturación de unidades de investigación competitivas do SUG do Plan Galego IDT, Ambiosol Group (ref. ED431C; 2022/40)).
Author information
Authors and Affiliations
Contributions
MAVR, SdaR, EUW, GNN, and XLO conceived the present manuscript. MAV, SdaR, EUW, JdaC, and AZdeS carried out soil and biota analysis; MAVR, XLO, SdaR, EUW, JdaC, and AZdeS participated in sample collection. All authors contributed to interpreting the results and writing the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ramos, M.A.V., da Rocha, S.S., Winkaler, E.U. et al. Soil Contamination and Biomarkers in Ucides cordatus in Mangroves from Baía de Todos os Santos, Bahia, Brazil. Water Air Soil Pollut 235, 218 (2024). https://doi.org/10.1007/s11270-024-07037-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-024-07037-0