Abstract
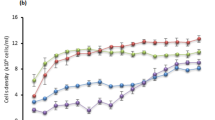
Tannins are special plant metabolites used in leather processing that act as pollutants. These substances are toxic to aquatic biota and can cause cell rupture. These harmful effects make the treatment of tannery wastewater difficult. Phytoplankton species are community components that are rarely considered in the biodegradation of organic compounds. However, in association with bacteria, these organisms can improve the biodegradation of pollutants by different mechanisms. The aim of the present study was to evaluate the potential of non-axenic cultures of Chlorella vulgaris containing Lactobacillus casei and Synechococcus sp. containing Rhizobium rosettiformans and Sphingomonas koreensis to biodegrade tannic acid (TA). Cultures in BG-11 medium containing TA (250 mg L−1) were incubated under a photoperiod or in the dark and monitored for 96 h. The cultures with added TA grew more than the control cultures under both the photoperiod and dark conditions. A reduction in the TA concentration and the TA metabolite gallic acid was observed under both conditions. Ellagic acid was identified and demonstrated resistance to biodegradation under the evaluated conditions, and neither of the other metabolites was detected. BG-11 culture medium is poor in organic material; therefore, microalgae and cyanobacteria contribute to bacterial metabolism. Under experimental conditions, phytoplankton species seem to contribute to the biodegradation of tannin residues, and in natural environments, they may aid in the bioremediation of sites contaminated by these pollutants.






Similar content being viewed by others
References
Abrusci, C., Palomar, J., Pablos, J. L., Rodriguez, F., & Catalina, F. (2011). Efficient biodegradation of common ionic liquids by Sphingomonas paucimobilis bacterium. Green Chemistry, 13, 709–717.
Aguilar, C. N., & Gutiérrez-Sánches, G. (2001). Review: Sources, properties, applications and potential uses of tannin acyl hydrolases. Food Science and Technology International, 7(5), 373–382.
Allen, M. M., & Stanier, R. Y. (1968). Selective isolation of blue-green algae from water and soil. Journal of General Microbiology, 51, 203–209.
Bergman, B., Zheng, W.-W., Klint, J., & Ran, L. (2008). On the origin of plants and relations to contemporary cyanobacterial-plant symbioses. Plant Biotechnology, 25, 213–220.
Casamatta, D. A., & Wickstrom, C. E. (2000). Sensitivity of two disjunct bacterioplankton communities to exudates from the cyanobacterium Microcystis aeruginosa Kützing. Microbial Ecology, 40(1), 64–73.
Christoff, A. P., Sereia, A. F. R., Boberg, D. R, Moraes, R. L., Oliveira, L. F. V. (2017). Bacterial identification through accurate library preparation and high-throughput sequencing. White paper: Bacterial NGS sequencing. https://neoprospecta.s3.amazonaws.com/docs/Neoprospecta+-+White+Paper+-+Bacterial+NGS+sequencing+2017.pdf
Divya, M., Aanand, S., Srinivasan, A., & Ahilan, B. (2015). Bioremediation – An eco-friendly tool for effluent treatment: A review. International Journal of Applied Research, 1(12), 530–537.
Dong-Hyeon, S., Kim, D., Seong, C., Song, H., & Ka, J. (2012). Genetic and phenotypic diversity of Carbofuran-degrading Bacteria isolated from agricultural soils. Journal of Microbiology and Biotechnology, 22(4), 448–456.
Dziallas, C., & Grossart, H. P. (2011). Temperature and biotic factors influence bacterial communities associated with Microcystis sp.(cyanobacteria). Environmental Microbiology, 13(6), 1632–1641.
El-Bestawy, E. A., El-Salam, A. Z. A., & Mansy, A. E. H. (2007). Potencial use of environmental cyanobacterial species in bioremediation of lindane-contaminated effluents. International Biodeterioration and Biodegradation, 59(3), 180–192.
Ferrera, I., & Sánchez, O. (2016). Insights into microbial diversity in wastewater treatment systems: How far have we come? Biotechnology Advances, 34(5), 790–802.
Franco, M. W., Ferreira, F. A. G., Vasconcelos, F. I., Batista, B. L., Pujoni, D. G. F., Magalhaes, S. M. S., Barbosa, F., Jr., & Barbosa, F. A. R. (2015). Arsenic biotransformation by cyanobacteria from mining areas: Evidences from culture experiments. Environmental Science and Pollution Research International, 22, 18607–18615.
Fuentes, J. F., Garbayo, I., Cuaresma, M., Montero, Z., González-del-Valle, M., & Vílchez, C. (2016). Impact of microalgae-Bacteria interactions on the production of algal biomass and associated compounds. Marine Drugs, 14, 100. https://doi.org/10.3390/md14050100.
Gauri, S. S., Mandal, S. M., Atta, S., Dey, S., & Pati, B. R. (2013). Novel route of tannic acid biotransformation and their effect on major biopolymer synthesis in Azotobacter sp. SSB81. Journal of Applied Microbiology, 114, 84–95.
Ghasemi, Y., Rasoul-Amini, S., & Fotooh-Abadi, E. (2011). The biotransformation, biodegradation, and bioremediation of organic compounds by microalgae. Journal of Phycology, 47(5), 969–980.
Gonçalves, A. L., Pires, J., M, C., & Simões, M. (2017). A review on the use of microalgal consortia for wastewater treatment. Algal Research, 24, 403–415.
Grossart, H. P., Kiørboe, T., Tang, K. W., Allgaier, M., Yam, E. M., & Ploug, H. (2006). Interactions between marine snow and heterotrophic bacteria: Aggregate formation and microbial dynamics. Aquatic Microbial Ecology, 42, 19–26.
Guo, P., Liu, Y., & Liu, C. (2015). Effects of chitosan, gallic acid, and algicide on the physiological and biochemical properties of Microcystis flos-aquae. Environmental Science and Pollution Research International, 22(17), 13514–13521.
He, Q., Yao, K., Sun, D., & Shi, B. (2007). Biodegradability of tannin-containing wastewater from leather industry. Biodegradation, 18(4), 465–472.
Huang, X., Shi, J., Cui, C., Yin, H., Zhang, R., Ma, X., & Zhang, X. (2016). Biodegradation of phenanthrene by rhizobium petrolearium SL-1. Journal of Applied Microbiology, 121(6), 1616–1626. https://doi.org/10.1111/jam.13292
Krezanoski, J. Z. (1966). Tannic acid: Chemistry, analysis, and toxicology. An episode in the pharmaceutics of radiology. Radiology., 87(4), 655–657.
Kumar, R. A., Gunasekaran, P., & Lakshmanan, M. (1999). Biodegradation of tannic acid by Citrobacter freundii isolated from a tannery effluent. Journal of Basic Microbiology, 39(3), 161–168.
Kuritz, T., & Wolk, C. P. (1995). Use of filamentous cyanobacteria for biodegradation of organic pollutants. Applied and Environmental Microbiology, 61(1), 234–238.
Laue, P., Bährs, H., Chakrabarti, S., & Steinberg, C. E. (2014). Natural xenobiotics to prevent cyanobacterial and algal growth in freshwater: Contrasting efficacy of tannic acid, gallic acid, and gramine. Chemosphere, 104, 212–220.
Lekha, P. K., & Lonsane, B. K. (1997). Production and application of tannin acyl hydrolase: State of the art. Advances in Applied Microbiology, 44, 215–260.
Lu, B. L., Qi, L., & Li, M. X. (2013). Effects of temperature on the growth and product accumulationof Chlorella sp. Advanced Materials Research, 712–715, 428–432.
Ma, J., Lu, N., Qin, W., Xu, R., Wang, Y., & Chen, X. (2006). Differential responses of eight cyanobacterial and green algal species to carbamate insecticides. Ecotoxicology and Environmental Safety, 63(2), 268–274.
Mingshu, L., Kay, Y., Qiang, H., & Dongying, J. (2006). Biodegradation of gallotannins and ellagitannins. Journal of Basic Microbiology, 46(1), 68–84.
Muñoz, R., & Guieysse, B. (2006). Algal-bacterial processes for the treatment of hazardous contaminants: A review. Water Research, 40(15), 2799–2815.
Nogales, J., Canales, A., Jimenez-Barbero, J., Serra, B., Pingarron, J. M., Garcia, J. L., & Diaz, E. (2011). Unravelling the gallic acid degradation pathway in bacteria: The gal cluster from Pseudomonas putida. Molecular Microbiology, 79(2), 359–374.
Osawa, R., Kuroiso, K., Goto, S., & Shimizu, A. (2000). Isolation of tannin-degrading lactobacilli from humans and fermented foods. Applied and Environmental Microbiology, 66(7), 3093–3097.
Osawa, R., Fujisawa, T., & Pukall, R. (2006). Lactobacillus apodemi sp. nov., a tannase-producing species isolated from wild mouse faeces. International Journal of Systematic and Evolutionary Microbiology, 56(Pt 7, 1693–1696.
Palanisami, S., Kannan, K., & Lakshmanan, U. (2012). Tannase activity from the marine cyanobacterium Phormidium valderianum BDU140441. Journal of Applied Phycology, 24(5), 1093–1098.
Pesce, S. F., & Wunderlin, D. A. (2004). Biodegradation of lindane by a native bacterial consortium isolated from contaminated river sediment. International Biodeterioration & Biodegradation, 54, 255–260.
Pinto, G., Pollio, A., Previtera, L., & Temussi, F. (2002). Biodegradation of phenols by microalgae. Biotechnology Letters, 24(24), 2047–2051.
R Development Core Team (2015). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/
Ramanan, R., Kim, B.-H., Cho, D.-Y., Oh, H.-M., & Kim, H. S. (2016). Algae–bacteria interactions: Evolution, ecology and emerging applications. Biotechnology Advances, 34(1), 14–29.
Rodriguez, H., De las Rivas, B., Gómez-Cordovés, C., & Muñoz, R. (2008). Characterization of tannase activity in cell-free extracts of Lactobacillus plantarum CECT 748T. International Journal of Food Microbiology, 121(1), 92–98.
Romero-Dondiz, E. M., Almazán, J. E., Rajal, V. B., & Castro-Vidaurre, E. F. (2015). Removal of vegetable tannins to recover water in the leather industry by ultrafiltration polymeric membranes. Chemical Engineering and Research Design, 93, 727–735.
Sasaki, E., Shimada, T., Osawa, R., Nishitani, Y., Spring, S., & Lang, E. (2005). Isolation of tannin-degrading bacteria isolated from feces of the Japanese large wood mouse, Apodemus speciosus, feeding on tannin-rich acorns. Systematic and Applied Microbiology, 28(4), 358–365.
Saxena, G., Chandra, R., & Bharagava, R. N. (2017). Environmental pollution, toxicity profile and treatment approaches for tannery wastewater and its chemical pollutants. Reviews of Environmental Contamination and Toxicology, 240, 31–69.
Seesuriyachana, P., Takenakab, S., Kuntiyaa, A., Klayraungc, S., Murakamib, S., & Aokib, K. (2007). Metabolism of azo dyes by Lactobacillus casei TISTR 1500 and effects of various factors on decolorization. Water Research, 41, 985–992.
Sena, L., Rojas, D., Montiel, E., González, H., Moret, J., & Naranjo, L. A. (2011). Strategy to obtain axenic cultures of Arthrospira spp. cyanobacteria. World Journal of Microbiology and Biotechnology, 27(5), 1045–1053.
Sepúlveda, M., Oliva, D., Urra, A., Pérez-Álvarez, M. J., Moraga, R., Schrader, D., Inostroza, P., Melo, A., Díaz, H., & Sielfeld, W. (2011). Distribution and abundance of the south American sea lion Otaria flavescens (Carnivora: Otariidae) along the central coast off Chile. Revista Chilena de Historia Natural, 84, 97–106.
Sharma, S., Bhat, T. K., & Dawra, R. K. (2000). A spectrophotometric method for assay of tannase using rhodanine. Analytical Biochemistry, 279(1), 85–89.
Subashchandrabose, S. R., Ramakrishnan, B., Megharaj, M., Venkateswarlu, K., & Naidu, R. (2013). Mixotrophic cyanobacteria and microalgae as distinctive biological agents for organic pollutant degradation. Environment International, 51, 59–72.
Surkatti, R., & Al-Zuhair, S. (2018). Microalgae cultivation for phenolic compounds removal. Environmental Science and Pollution Research, 25(34), 33936–33956.
Tang, W. J., Zhang, L. S., Fang, Y., Zhou, Y., & Ye, B. C. (2016). Biodegradation of phthalate esters by newly isolated rhizobium sp. LMB-1 and its biochemical pathway of di-n-butyl phthalate. Journal of Applied Microbiology, 121(1), 177–186.
Acknowledgments
The authors thank Professor Gecernir Colen for his suggestions and discussion during this study and the Chemistry Department of the Institute of Exact Sciences of the Federal University of Minas Gerais for conducting the chemical analysis.
Funding
This study was funded by the Minas Gerais State Agency for Research and Development (project number CBB-APQ-01491-11).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Silva, S.B., Pádua, R.M., Barbosa, F.A.R. et al. Phytoplankton Cultures for Tannin Biodegradation. Water Air Soil Pollut 230, 170 (2019). https://doi.org/10.1007/s11270-019-4199-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-019-4199-5