Abstract
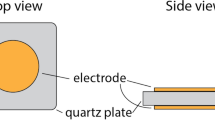
A polyaniline-modified quartz crystal microbalance (QCM) sensor was obtained through immobilizing the polyaniline film on the silver electrode surface of quartz crystal resonator by an electrochemical method. The sensor was studied for detecting the formic acid gas of different concentrations, and the results showed that the resonant frequency of QCM decreased quickly in the beginning and tended to be constant in the end when exposed to formic acid gas. The frequency shifts decreased faster as the concentration of formic acid gas increased. And the frequency shifts of the QCM sensors were found to be linearly related to the concentration of formic acid gas, which might be used to estimate the concentration level of the formic acid gas within the range of experimental concentrations. The result of on-line monitoring test fully indicated that the QCM sensor responded effectively to the increasing concentration of formic acid and had important practical significance and broad application prospect in real-time detection of antique conservation environment in the museum.






Similar content being viewed by others
References
Adhikari, B., & Majumdar, S. (2004). Polymers in sensor applications. Progress in Polymer Science, 29(7), 699–766.
Cruz, A. J., Pires, J., & Carvalho, A. P. (2008). Comparison of adsorbent materials for acetic acid removal in showcase. Journal of Cultural Heritage, 9(3), 244–252.
Du, Z. F., Li, C. C., & Li, L. M. (2011). Ammonia gas detection based on polyaniline nanofibers coated on interdigitated array electrodes. Journal of Materials Science: Materials in Electronics, 22(4), 418–421.
Fang, H. Q., Li, G. X., & Chen, H. Y. (1994). Polyaniline modified electrode prepared by galvanostatic at low current density. Chemical Journal of Chinese Universities, 15(3), 348–351.
Fench, A., Strlic, M., Cigic, I. K., et al. (2010). Volatile aldehydes in libraries and archives. Atmospheric Environment, 44(17), 2067–2073.
Grate, J. W. (2000). Acoustic wave microsensor arrays for vapor sensing. Chemical Reviews, 100, 2627–2648.
Harbeck, M., Tasaltin, C., Gurol, I., et al. (2010). Preferential sorption of polar compounds by fluoroalkyloxy substituted phthalocyanines for the use in sorption based gas sensors. Sensors and Actuators B, 150(2), 616–624.
Khan, A. A., Baig, U., & Khalid, M. (2011). Ammonia vapor sensing properties of polyaniline-titanium(IV) phosphate cation exchange nanocomposite. Journal of Hazardous Materials, 186(2–3), 2037–2042.
Li, X. X., Ju, M., & Li, X. W. (2004). Chlorine ion sensor based on polyaniline film electrode. Sensors and Actuators B, 97, 144–147.
Liu, B. Y., Chen, X. J., Fang, D. Y., et al. (2010). Environmental monitoring by thin film nanocomposite sensors for cultural heritage preservation. Journal of alloys and compounds, 504(suppl.1), s405–s409.
Neri, A., Corbellini, S., Parvis, M., et al, (2009). Environmental monitoring of heritage buildings. 2009 IEEE Workshop on Environmental Energy, and Structural Monitoring Systems, 93–97.
Nicolas-Debarnot, D., & Poncin-Epaillard, F. (2003). Polyaniline as a new sensitive layer for gas sensors. Analytica Chimica Acta, 475(1–2), 1–15.
Oikawa, T. M., Matsuda, Y., et al. (2005). Volatile organic compounds from woods and their influences on museum artifact material I: differences in wood species and analyses of casual substances of deterioration. The Japan Wood Research Society, 51(4), 363–369.
Ryhl-Svendsen, M., & Glastrap, J. (2002). Acetic acid and formic acid concentrations in the museum environment measured by SPME-GC/Ms. Atmospheric Environment, 36(24), 3909–3916.
Wang, X.H., Li, M., Chen, S.Y. (2011). Long memory from Sauerbrey equation: a case in coated quartz crystal microbalance in terms of ammonia. Mathematical Problems in Engineering, 1–9.
Wu, X. Q., Takao, Y., & Egashira, M. (1997). Polyaniline-modified quartz crystal microbalance gas sensor. Electrochemistry, 3(3), 330–333.
Xu, X. M., Cang, H. W., Li, C. Z., et al. (2009). Quartz crystal microbalance sensor array for the detection of volatile organic compounds. Talanta, 78(3), 711–716.
Xu, X. M., Li, C. Z., Pei, K. M., et al. (2008). Ionic liquids used as QCM coating materials for the detection of alcohols. Sensors and Actuators B, 134, 258–265.
Acknowledgments
This work was supported by the National Rolling Project of Scientific and Technical Supporting Programs Funded by Ministry of Science & Technology of China (2010BAK67B15), the Project of Natural Science Foundation of Shanghai City (10ZR1407900), and the Opening Project of Shanghai Museum (2008001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yan, Y., Lu, D., Zhou, H. et al. Polyaniline-Modified Quartz Crystal Microbalance Sensor for Detection of Formic Acid Gas. Water Air Soil Pollut 223, 1275–1280 (2012). https://doi.org/10.1007/s11270-011-0943-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-011-0943-1