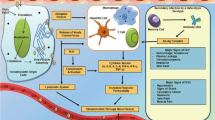
Abstract
Structural genomics involves the advent of three-dimensional structures of the genome encoded proteins through various techniques available. Numerous structural genomics research groups have been developed across the globe and they contribute enormously to the identification of three-dimensional structures of various proteins. In this review, we have discussed the applications of the structural genomics approach towards the discovery of potential lead-like molecules against the genomic drug targets of three vector-borne diseases, namely, Dengue, Chikungunya and Zika. Currently, all these three diseases are associated with the most important global public health problems and significant economic burden in tropical countries. Structural genomics has accelerated the identification of novel drug targets and inhibitors for the treatment of these diseases. We start with the current development status of the drug targets and antiviral drugs against these three diseases and conclude by describing challenges that need to be addressed to overcome the shortcomings in the process of drug discovery.







Similar content being viewed by others
References
Weigelt J, McBroom-Cerajewski LD, Schapira M, Zhao Y, Arrowmsmith CH (2008) Structural genomics and drug discovery: all in the family. Curr Opin Chem Biol 12(1):32–39
Stevens RC, Yokoyama S, Wilson IA (2001) Global efforts in structural genomics. Science 294(5540):89–92
Lundstrom K (2006) Structural genomics for membrane proteins. Cell Mol Life Sci 63(22):2597–2607
Lundstrom K (2005) Structural genomics of GPCRs. Trends Biotechnol 23(2):103–108
Marsden BD, Knapp S (2008) Doing more than just the structure—structural genomics in kinase drug discovery. Curr Opin Chem Biol 12(1):40–45
https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases
Zanotto PM, Leite LC (2018) The challenges imposed by Dengue, Zika, and Chikungunya to Brazil. Front Immunol. https://doi.org/10.3389/fimmu.2018.01964
Guzman MG, Halstead SB, Artsob H, Buchy P, Farrar J, Gubler DJ, Hunsperger E, Kroeger A, Margolis HS, Martínez E, Nathan MB (2010) Dengue: a continuing global threat. Nat Rev Microbiol 8(12):S7-16
Modis Y, Ogata S, Clements D, Harrison SC (2003) A ligand-binding pocket in the dengue virus envelope glycoprotein. Proc Natl Acad Sci 100(12):6986–6991
Xia H, Xie X, Zou J, Noble CG, Russell WK, Holthauzen LM, Choi KH, White MA, Shi PY (2020) A cocrystal structure of dengue capsid protein in complex of inhibitor. Proc Natl Acad Sci 117(30):17992–18001
Li L, Lok SM, Yu IM, Zhang Y, Kuhn RJ, Chen J, Rossmann MG (2008) The flavivirus precursor membrane-envelope protein complex: structure and maturation. Science 319(5871):1830–1834
Byrd CM, Dai D, Grosenbach DW, Berhanu A, Jones KF, Cardwell KB, Schneider C, Wineinger KA, Page JM, Harver C, Stavale E (2013) A novel inhibitor of dengue virus replication that targets the capsid protein. Antimicrob Agents Chemother 57(1):15–25
Smith JL, Sheridan K, Parkins CJ, Frueh L, Jemison AL, Strode K, Dow G, Nilsen A, Hirsch AJ (2018) Characterization and structure-activity relationship analysis of a class of antiviral compounds that directly bind dengue virus capsid protein and are incorporated into virions. Antiviral Res 155:12–19
Schmidt AG, Lee K, Yang PL, Harrison SC (2012) Small-molecule inhibitors of dengue-virus entry. PLoS Pathog 8(4):e1002627
Yang JM, Chen YF, Tu YY, Yen KR, Yang YL (2007) Combinatorial computational approaches to identify tetracycline derivatives as flavivirus inhibitors. PLoS ONE 2(5):e428
Poh MK, Yip A, Zhang S, Priestle JP, Ma NL, Smit JM, Wilschut J, Shi PY, Wenk MR, Schul W (2009) A small molecule fusion inhibitor of dengue virus. Antiviral Res 84(3):260–266
Leal ES, Adler NS, Fernández GA, Gebhard LG, Battini L, Aucar MG, Videla M, Monge ME, de Los Ríos AH, Dávila JA, Morell ML (2019) De novo design approaches targeting an envelope protein pocket to identify small molecules against dengue virus. Eur J Med Chem 182:111628
Zhou Z, Khaliq M, Suk JE, Patkar C, Li L, Kuhn RJ, Post CB (2008) Antiviral compounds discovered by virtual screening of small-molecule libraries against dengue virus E protein. ACS Chem Biol 3(12):765–775
Kato D, Era S, Watanabe I, Arihara M, Sugiura N, Kimata K, Suzuki Y, Morita K, Hidari KI, Suzuki T (2010) Antiviral activity of chondroitin sulphate E targeting dengue virus envelope protein. Antiviral Res 88(2):236–243
Lin YL, Lei HY, Lin YS, Yeh TM, Chen SH, Liu HS (2002) Heparin inhibits dengue-2 virus infection of five human liver cell lines. Antiviral Res 56(1):93–96
Hidari KI, Takahashi N, Arihara M, Nagaoka M, Morita K, Suzuki T (2008) Structure and anti-dengue virus activity of sulfated polysaccharide from a marine alga. Biochem Biophys Res Commun 376(1):91–95
Talarico LB, Damonte EB (2007) Interference in dengue virus adsorption and uncoating by carrageenans. Virology 363(2):473–485
Lee E, Pavy M, Young N, Freeman C, Lobigs M (2006) Antiviral effect of the heparan sulfate mimetic, PI-88, against dengue and encephalitic flaviviruses. Antiviral Res 69(1):31–38
Lin LT, Chen TY, Lin SC, Chung CY, Lin TC, Wang GH, Anderson R, Lin CC, Richardson CD (2013) Broad-spectrum antiviral activity of chebulagic acid and punicalagin against viruses that use glycosaminoglycans for entry. BMC Microbiol 13(1):1–5
Faustino AF, Guerra GM, Huber RG, Hollmann A, Domingues MM, Barbosa GM, Enguita FJ, Bond PJ, Castanho MA, Da Poian AT, Almeida FC (2015) Understanding dengue virus capsid protein disordered N-terminus and pep14-23-based inhibition. ACS Chem Biol 10(2):517–526
Balinsky CA, Schmeisser H, Ganesan S, Singh K, Pierson TC, Zoon KC (2013) Nucleolin interacts with the dengue virus capsid protein and plays a role in formation of infectious virus particles. J Virol 87(24):13094–13106
Qin CF, Qin ED (2004) Development of cell lines stably expressing staphylococcal nuclease fused to dengue 2 virus capsid protein for CTVI. Acta Biochim Biophys Sin 36(8):577–582
Schmidt AG, Yang PL, Harrison SC (2010) Peptide inhibitors of dengue-virus entry target a late-stage fusion intermediate. PLoS Pathog 6(4):e1000851
Hrobowski YM, Garry RF, Michael SF (2005) Peptide inhibitors of dengue virus and West Nile virus infectivity. Virology J 2(1):1
Isa DM, Chin SP, Chong WL, Zain SM, Abd Rahman N, Lee VS (2019) Dynamics and binding interactions of peptide inhibitors of dengue virus entry. J Biol Phys 45(1):63–76
Costin JM, Jenwitheesuk E, Lok SM, Hunsperger E, Conrads KA, Fontaine KA, Rees CR, Rossmann MG, Isern S, Samudrala R, Michael SF (2010) Structural optimization and de novo design of dengue virus entry inhibitory peptides. PLoS Negl Trop Dis 4(6):e721
Panya A, Sawasdee N, Junking M, Srisawat C, Choowongkomon K, Yenchitsomanus PT (2015) A peptide inhibitor derived from the conserved ectodomain region of DENV membrane (M) protein with activity against dengue virus infection. Chem Biol Drug Des 86(5):1093–1104
Edeling MA, Diamond MS, Fremont DH (2014) Structural basis of Flavivirus NS1 assembly and antibody recognition. Proc Natl Acad Sci 111(11):4285–4290
Libraty DH, Young PR, Pickering D, Endy TP, Kalayanarooj S, Green S et al (2002) High circulating levels of the dengue virus nonstructural protein NS1 early in dengue illness correlate with the development of dengue hemorrhagic fever. J Infect Dis 186(8):1165–1168. https://doi.org/10.1086/343813
Songprakhon P, Thaingtamtanha T, Limjindaporn T, Puttikhunt C, Srisawat C, Luangaram P, Dechtawewat T, Uthaipibull C, Thongsima S, Yenchitsomanus PT, Malasit P (2020) Peptides targeting dengue viral nonstructural protein 1 inhibit dengue virus production. Sci Rep 10(1):1–6
Chandramouli S, Joseph JS, Daudenarde S, Gatchalian J, Cornillez-Ty C, Kuhn P (2010) Serotype-specific structural differences in the protease-cofactor complexes of the dengue virus family. J Virol 84(6):3059–3067
Rothan HA, Han HC, Ramasamy TS, Othman S, Abd Rahman N, Yusof R (2012) Inhibition of dengue NS2B-NS3 protease and viral replication in Vero cells by recombinant retrocyclin-1. BMC Infect Dis 12(1):1–9
Yang CC, Hu HS, Wu RH, Wu SH, Lee SJ, Jiaang WT, Chern JH, Huang ZS, Wu HN, Chang CM, Yueh A (2014) A novel dengue virus inhibitor, BP13944, discovered by high-throughput screening with dengue virus replicon cells selects for resistance in the viral NS2B/NS3 protease. Antimicrob Agents Chemother 58(1):110–119
Tomlinson SM, Malmstrom RD, Russo A, Mueller N, Pang YP, Watowich SJ (2009) Structure-based discovery of dengue virus protease inhibitors. Antiviral Res 82(3):110–114
Matusan AE, Pryor MJ, Davidson AD, Wright PJ (2001) Mutagenesis of the Dengue virus type 2 NS3 protein within and outside helicase motifs: effects on enzyme activity and virus replication. J Virol 75(20):9633–9643
Swarbrick CM, Basavannacharya C, Chan KW, Chan SA, Singh D, Wei N, Phoo WW, Luo D, Lescar J, Vasudevan SG (2017) NS3 helicase from dengue virus specifically recognizes viral RNA sequence to ensure optimal replication. Nucleic Acids Res 45(22):12904–12920
Mastrangelo E, Pezzullo M, De Burghgraeve T, Kaptein S, Pastorino B, Dallmeier K, de Lamballerie X, Neyts J, Hanson AM, Frick DN, Bolognesi M (2012) Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: new prospects for an old drug. J Antimicrob Chemother 67(8):1884–1894
Byrd CM, Grosenbach DW, Berhanu A, Dai D, Jones KF, Cardwell KB, Schneider C, Yang G, Tyavanagimatt S, Harver C, Wineinger KA (2013) Novel benzoxazole inhibitor of dengue virus replication that targets the NS3 helicase. Antimicrob Agents Chemother 57(4):1902–1912
Basavannacharya C, Vasudevan SG (2014) Suramin inhibits helicase activity of NS3 protein of dengue virus in a fluorescence-based high throughput assay format. Biochem Biophys Res Commun 453(3):539–544
Norazharuddin H, Lai NS (2018) Roles and prospects of dengue virus non-structural proteins as antiviral targets: an easy digest. Malays J Med Sci 25(5):6
van Cleef KW, Overheul GJ, Thomassen MC, Marjakangas JM, van Rij RP (2016) Escape mutations in NS4B render dengue virus insensitive to the antiviral activity of the paracetamol metabolite AM404. Antimicrob Agents Chemother 60(4):2554–2557
Xie X, Wang QY, Xu HY, Qing M, Kramer L, Yuan Z, Shi PY (2011) Inhibition of dengue virus by targeting viral NS4B protein. J Virol 85(21):11183–11195
Nguyen NM, Tran CN, Phung LK, Duong KT, Huynh HL, Farrar J, Nguyen QT, Tran HT, Nguyen CV, Merson L, Hoang LT (2013) A randomized, double-blind placebo controlled trial of balapiravir, a polymerase inhibitor, in adult dengue patients. J Infect Dis 207(9):1442–1450
Carocci M, Hinshaw SM, Rodgers MA, Villareal VA, Burri DJ, Pilankatta R, Maharaj NP, Gack MU, Stavale EJ, Warfield KL, Yang PL (2015) The bioactive lipid 4-hydroxyphenyl retinamide inhibits flavivirus replication. Antimicrob Agents Chemother 59(1):85–95
Yokokawa F, Nilar S, Noble CG, Lim SP, Rao R, Tania S, Wang G, Lee G, Hunziker J, Karuna R, Manjunatha U (2016) Discovery of potent non-nucleoside inhibitors of dengue viral RNA-dependent RNA polymerase from a fragment hit using structure-based drug design. J Med Chem 59(8):3935–3952
Lim SP, Noble CG, Nilar S, Shi PY, Yokokawa F (2018) Discovery of potent non-nucleoside inhibitors of dengue viral RNA-dependent RNA polymerase from fragment screening and structure-guided design. Dengue Zika: Control Antivir Treat Strateg. https://doi.org/10.1007/978-981-10-8727-1_14
Benmansour F, Trist I, Coutard B, Decroly E, Querat G, Brancale A, Barral K (2017) Discovery of novel dengue virus NS5 methyltransferase non-nucleoside inhibitors by fragment-based drug design. Eur J Med Chem 125:865–880
Haese N, Powers J, Streblow DN (2020) Small molecule inhibitors targeting chikungunya virus. Chikungunya Virus. https://doi.org/10.1007/82_2020_195
Sharma R, Fatma B, Saha A, Bajpai S, Sistla S, Dash PK, Parida M, Kumar P, Tomar S (2016) Inhibition of chikungunya virus by picolinate that targets viral capsid protein. Virology 498:265–276
Kumar R, Nehul S, Singh A, Tomar S (2021) Identification and evaluation of antiviral potential of thymoquinone, a natural compound targeting Chikungunya virus capsid protein. Virology 561:36–46
Sharma R, Kesari P, Kumar P, Tomar S (2018) Structure-function insights into chikungunya virus capsid protein: small molecules targeting capsid hydrophobic pocket. Virology 515:223–234
Fatma B, Kumar R, Singh VA, Nehul S, Sharma R, Kesari P, Kuhn RJ, Tomar S (2020) Alphavirus capsid protease inhibitors as potential antiviral agents for Chikungunya infection. Antiviral Res 179:104808
Weber C, Berberich E, von Rhein C, Henß L, Hildt E, Schnierle BS (2017) Identification of functional determinants in the chikungunya virus E2 protein. PLoS Negl Trop Dis 11(1):e0005318
Uchime O, Fields W, Kielian M (2013) The role of E3 in pH protection during alphavirus assembly and exit. J Virol 87(18):10255–10262
Voss JE, Vaney MC, Duquerroy S, Vonrhein C, Girard-Blanc C, Crublet E, Thompson A, Bricogne G, Rey FA (2010) Glycoprotein organization of Chikungunya virus particles revealed by X-ray crystallography. Nature 468(7324):709–712
Nguyen PT, Yu H, Keller PA (2018) Molecular docking studies to explore potential binding pockets and inhibitors for chikungunya virus envelope glycoproteins. Interdiscip Sci: Comput Life Sci 10(3):515–524
Passos GF, Gomes MG, Aquino TM, Araújo-Júnior JX, Souza SJ, Cavalcante JP, Santos EC, Bassi ÊJ, Silva-Júnior EF (2020) Computer-aided design, synthesis, and antiviral evaluation of novel acrylamides as potential inhibitors of E3-E2-E1 glycoproteins complex from Chikungunya virus. Pharmaceuticals 13(7):141
Islamuddin M, Afzal O, Khan WH, Hisamuddin M, Altamimi AS, Husain I, Kato K, Alamri MA, Parveen S (2021) Inhibition of Chikungunya Virus Infection by 4-Hydroxy-1-Methyl-3-(3-morpholinopropanoyl) quinoline-2 (1 H)-one (QVIR) Targeting nsP2 and E2 Proteins. ACS Omega 6(14):9791–9803
Dey D, Siddiqui SI, Mamidi P, Ghosh S, Kumar CS, Chattopadhyay S, Ghosh S, Banerjee M (2019) The effect of amantadine on an ion channel protein from Chikungunya virus. PLoS Negl Trop Dis 13(7):e0007548
Rupp JC, Sokoloski KJ, Gebhart NN, Hardy RW (2015) Alphavirus RNA synthesis and non-structural protein functions. J Gen Virol 96(Pt 9):2483
Feibelman KM, Fuller BP, Li L, LaBarbera DV, Geiss BJ (2018) Identification of small molecule inhibitors of the Chikungunya virus nsP1 RNA capping enzyme. Antivir Res 154:124–131
Gigante A, Canela MD, Delang L, Priego EM, Camarasa MJ, Querat G, Neyts J, Leyssen P, Pérez-Pérez MJ (2014) Identification of [1, 2, 3] triazolo [4, 5-d] pyrimidin-7 (6 H)-ones as novel inhibitors of Chikungunya virus replication. J Med Chem 57(10):4000–4008
Mudgal R, Mahajan S, Tomar S (2020) Inhibition of Chikungunya virus by an adenosine analog targeting the SAM-dependent nsP1 methyltransferase. FEBS Lett 594(4):678–694
Kovacikova K, Morren BM, Tas A, Albulescu IC, van Rijswijk R, Jarhad DB, Shin YS, Jang MH, Kim G, Lee HW, Jeong LS (2020) 6′-β-Fluoro-homoaristeromycin and 6′-fluoro-homoneplanocin A are potent inhibitors of chikungunya virus replication through their direct effect on viral nonstructural protein 1. Antimicrob Agents Chemother 64(4):e02532-e2619
Moesslacher J, Battisti V, Delang L, Neyts J, Abdelnabi R, Pürstinger G, Urban E, Langer T (2020) Identification of 2-(4-(phenylsulfonyl) piperazine-1-yl) pyrimidine analogues as novel inhibitors of chikungunya virus. ACS Med Chem Lett 11(5):906–912
Zhang K, Law YS, Law MC, Tan YB, Wirawan M, Luo D (2021) Structural insights into viral RNA capping and plasma membrane targeting by Chikungunya virus nonstructural protein 1. Cell Host Microbe 29(5):757–764
Kovacikova K, Gorostiola González M, Jones R, Reguera J, Gigante A, Pérez-Pérez MJ, Pürstinger G, Moesslacher J, Langer T, Jeong LS, Delang L (2021) Structural insights into the mechanisms of action of functionally distinct classes of Chikungunya virus nonstructural protein 1 inhibitors. Antimicrob Agents Chemother 65(7):e02566-e2620
Fros JJ, van der Maten E, Vlak JM, Pijlman GP (2013) The C-terminal domain of chikungunya virus nsP2 independently governs viral RNA replication, cytopathicity, and inhibition of interferon signaling. J Virol 87(18):10394–10400
Narwal M, Singh H, Pratap S, Malik A, Kuhn RJ, Kumar P, Tomar S (2018) Crystal structure of chikungunya virus nsP2 cysteine protease reveals a putative flexible loop blocking its active site. Int J Biol Macromol 116:451–462
Bassetto M, De Burghgraeve T, Delang L, Massarotti A, Coluccia A, Zonta N, Gatti V, Colombano G, Sorba G, Silvestri R, Tron GC (2013) Computer-aided identification, design and synthesis of a novel series of compounds with selective antiviral activity against chikungunya virus. Antivir Res 98(1):12–18
Nguyen PT, Yu H, Keller PA (2015) Identification of chikungunya virus nsP2 protease inhibitors using structure-base approaches. J Mol Graph Model 57:1–8
Agarwal T, Asthana S, Bissoyi A (2015) Molecular modeling and docking study to elucidate novel chikungunya virus nsP2 protease inhibitors. Indian J Pharm Sci 77(4):453
Jain J, Kumari A, Somvanshi P, Grover A, Pai S, Sunil S (2017) In silico analysis of natural compounds targeting structural and nonstructural proteins of chikungunya virus. F1000Research 6:1601
Kumar P, Kumar D, Giri R (2019) Targeting the nsp2 cysteine protease of chikungunya virus using FDA approved library and selected cysteine protease inhibitors. Pathogens 8(3):128
Meena MK, Kumar D, Kumari K, Kaushik NK, Kumar RV, Bahadur I, Vodwal L, Singh P (2021) Promising inhibitors of nsp2 of CHIKV using molecular docking and temperature-dependent molecular dynamics simulations. J Biomol Struct Dyn. https://doi.org/10.1080/07391102.2021.1873863
Rasool N, Bakht A, Hussain W (2021) Analysis of inhibitor binding combined with reactivity studies to discover the potentially inhibiting phytochemicals targeting Chikungunya viral replication. Curr Drug Discov Technol 18(3):437–450
Lani R, Agharbaoui FE, Hassandarvish P, Teoh BT, Sam SS, Zandi K, Rahman NA, AbuBakar S (2021) In silico studies of fisetin and silymarin as novel chikungunya virus nonstructural proteins inhibitors. Future Virol 16(3):167–180
Singh Jadav S, Nayan Sinha B, Pastorino B, de Lamballerie X, Hilgenfeld R, Jayaprakash V (2015) Identification of pyrazole derivative as an antiviral agent against Chikungunya through HTVS. Lett Drug Des Discov 12(4):292–301
Das PK, Puusepp L, Varghese FS, Utt A, Ahola T, Kananovich DG, Lopp M, Merits A, Karelson M (2016) Design and validation of novel chikungunya virus protease inhibitors. Antimicrob Agents Chemother 60(12):7382–7395
Ivanova L, Rausalu K, Žusinaite E, Tammiku-Taul J, Merits A, Karelson M (2021) 1, 3-Thiazolbenzamide derivatives as Chikungunya virus nsP2 protease inhibitors. ACS Omega 6(8):5786–5794
Eberle RJ, Olivier DS, Pacca CC, Avilla CM, Nogueira ML, Amaral MS, Willbold D, Arni RK, Coronado MA (2021) In vitro study of Hesperetin and Hesperidin as inhibitors of zika and chikungunya virus proteases. PLoS ONE 16(3):e0246319
Tripathi PK, Soni A, Yadav SP, Kumar A, Gaurav N, Raghavendhar S, Sharma P, Sunil S, Jayaram B, Patel AK (2020) Evaluation of novobiocin and telmisartan for anti-CHIKV activity. Virology 548:250–260
Tripathi PK, Singh J, Gaurav N, Garg DK, Patel AK (2020) In-silico and biophysical investigation of biomolecular interaction between naringin and nsP2 of the chikungunya virus. Int J Biol Macromol 160:1061–1065
Singh H, Mudgal R, Narwal M, Kaur R, Singh VA, Malik A, Chaudhary M, Tomar S (2018) Chikungunya virus inhibition by peptidomimetic inhibitors targeting virus-specific cysteine protease. Biochimie 149:51–61
El-labbad EM, Ismail MA, Abou Ei Ella DA, Ahmed M, Wang F, Barakat KH, Abouzid KA (2015) Discovery of novel peptidomimetics as irreversible CHIKV Ns P 2 protease inhibitors using quantum mechanical-based ligand descriptors. Chem Biol Drug Des 86(6):1518–1527
Law YS, Utt A, Tan YB, Zheng J, Wang S, Chen MW, Griffin PR, Merits A, Luo D (2019) Structural insights into RNA recognition by the Chikungunya virus nsP2 helicase. Proc Natl Acad Sci 116(19):9558–9567
Abraham R, Hauer D, McPherson RL, Utt A, Kirby IT, Cohen MS, Merits A, Leung AK, Griffin DE (2018) ADP-ribosyl–binding and hydrolase activities of the alphavirus nsP3 macrodomain are critical for initiation of virus replication. Proc Natl Acad Sci 115(44):E10457–E10466
Malet H, Coutard B, Jamal S, Dutartre H, Papageorgiou N, Neuvonen M, Ahola T, Forrester N, Gould EA, Lafitte D, Ferron F (2009) The crystal structures of Chikungunya and Venezuelan equine encephalitis virus nsP3 macro domains define a conserved adenosine binding pocket. J Virol 83(13):6534–6545
Zhang S, Garzan A, Haese N, Bostwick R, Martinez-Gzegozewska Y, Rasmussen L, Streblow DN, Haise MT, Pathak AK, Augelli-Szafran CE, Wu M (2021) Pyrimidone inhibitors targeting Chikungunya Virus nsP3 macrodomain by fragment-based drug design. PLoS ONE 16(1):e0245013
Shimizu JF, Martins DO, McPhillie MJ, Roberts GC, Zothner C, Merits A, Harris M, Jardim AC (2020) Is the ADP ribose site of the Chikungunya virus NSP3 Macro domain a target for antiviral approaches? Acta Trop 207:105490
Nguyen PT, Yu H, Keller PA (2014) Discovery of in silico hits targeting the nsP3 macro domain of chikungunya virus. J Mol Model 20(5):1–2
Kumar D, Kumari K, Jayaraj A, Singh P (2020) Development of a theoretical model for the inhibition of nsP3 protease of Chikungunya virus using pyranooxazoles. J Biomol Struct Dyn 38(10):3018–3034
Meena MK, Kumar D, Jayaraj A, Kumar A, Kumari K, Katata-Seru LM, Bahadur I, Kumar V, Sherawat A, Singh P (2020) Designed thiazolidines: an arsenal for the inhibition of nsP3 of CHIKV using molecular docking and MD simulations. J Biomol Struct Dyn. https://doi.org/10.1080/07391102.2020.1832918
Kumar D, Meena MK, Kumari K, Patel R, Jayaraj A, Singh P (2020) In-silico prediction of novel drug-target complex of nsp3 of CHIKV through molecular dynamic simulation. Heliyon 6(8):e04720
Chaudhary M, Sehgal D (2021) In silico identification of natural antiviral compounds as a potential inhibitor of chikungunya virus non-structural protein 3 macrodomain. J Biomol Struct Dyn. https://doi.org/10.1080/07391102.2021.1960195
Patil P, Agrawal M, Almelkar S, Jeengar MK, More A, Alagarasu K, Kumar NV, Mainkar PS, Parashar D, Cherian S (2021) In vitro and in vivo studies reveal α-Mangostin, a xanthonoid from Garcinia mangostana, as a promising natural antiviral compound against chikungunya virus. Virol J 18(1):1–2
LaStarza MW, Lemm JA, Rice CM (1994) Genetic analysis of the nsP3 region of Sindbis virus: evidence for roles in minus-strand and subgenomic RNA synthesis. J Virol 68(9):5781–5791
Nandi I, Gupta A, Chaudhary VK, Gupta V, Gabrani R, Gupta S (2019) Expression, purification and functional characterization of recombinant hypervariable region (HVR) of Chikungunya virus nsP3 protein. 3 Biotech 9(6):1–8
Satheesh G, Prabhu PN, Venkataramana M (2014) 3D Modeling of dengue virus NS4B and Chikungunya virus nsP4: identification of a common drug target and designing a single antiviral inhibitor. Curr Comput Aided Drug Des 10(4):361–373
Reyes-Gastellou A, Jiménez-Alberto A, Castelán-Vega JA, Aparicio-Ozores G, Ribas-Aparicio RM (2021) Chikungunya nsP4 homology modeling reveals a common motif with Zika and Dengue RNA polymerases as a potential therapeutic target. J Mol Model 27(9):1–2
Wada Y, Orba Y, Sasaki M, Kobayashi S, Carr MJ, Nobori H, Sato A, Hall WW, Sawa H (2017) Discovery of a novel antiviral agent targeting the nonstructural protein 4 (nsP4) of chikungunya virus. Virology 505:102–112
Ehteshami M, Tao S, Zandi K, Hsiao HM, Jiang Y, Hammond E, Amblard F, Russell OO, Merits A, Schinazi RF (2017) Characterization of β-d-N 4-hydroxycytidine as a novel inhibitor of chikungunya virus. Antimicrob Agents Chemother 61(4):e02395-e2416
Ferreira AC, Reis PA, de Freitas CS, Sacramento CQ, Villas Bôas Hoelz L, Bastos MM, Mattos M, Rocha N, Gomes de Azevedo Quintanilha I, da Silva Gouveia Pedrosa C, Rocha Quintino Souza L (2019) Beyond members of the Flaviviridae family, sofosbuvir also inhibits chikungunya virus replication. Antimicrob Agents Chemother 63(2):e01389-18
Kumar SP, Kapopara RG, Patni MI, Pandya HA, Jasrai YT, Patel SK (2012) Exploring the polymerase activity of chikungunya viral non structural protein 4 (nsP4) using molecular modeling, epharmacophore and docking studies. Int J Pharm Life Sci 3(6):1752–1765
Ghildiyal R, Gupta S, Gabrani R, Joshi G, Gupta A, Chaudhary VK, Gupta V (2019) In silico study of chikungunya polymerase, a potential target for inhibitors. Virusdisease 30(3):394–402
Ayres CF, Guedes DR, Paiva MH, Morais-Sobral MC, Krokovsky L, Machado LC, Melo-Santos MA, Crespo M, Oliveira CM, Ribeiro RS, Cardoso OA (2019) Zika virus detection, isolation and genome sequencing through Culicidae sampling during the epidemic in Vitória, Espírito Santo, Brazil. Parasit Vectors 12(1):220
Shang Z, Song H, Shi Y, Qi J, Gao GF (2018) Crystal structure of the capsid protein from Zika virus. J Mol Biol 430(7):948–962
Ahmed SR, Banik A, Anni SM, Chowdhury MM (2020) Plant derived bioactive compounds as potential inhibitors of ZIKA virus: an in silico investigation. bioRxiv. https://doi.org/10.1101/2020.11.11.378083
Dai L, Song J, Lu X, Deng YQ, Musyoki AM, Cheng H, Zhang Y, Yuan Y, Song H, Haywood J, Xiao H (2016) Structures of the Zika virus envelope protein and its complex with a flavivirus broadly protective antibody. Cell Host Microbe 19(5):696–704
Fernando S, Fernando T, Stefanik M, Eyer L, Ruzek D (2016) An approach for Zika virus inhibition using homology structure of the envelope protein. Mol Biotechnol 58(12):801–806
Carneiro BM, Batista MN, Braga AC, Nogueira ML, Rahal P (2016) The green tea molecule EGCG inhibits Zika virus entry. Virology 496:215–218
Barrows NJ, Campos RK, Powell ST, Prasanth KR, Schott-Lerner G, Soto-Acosta R, Galarza-Muñoz G, McGrath EL, Urrabaz-Garza R, Gao J, Wu P (2016) A screen of FDA-approved drugs for inhibitors of Zika virus infection. Cell Host Microbe 20(2):259–270
Mounce BC, Cesaro T, Carrau L, Vallet T, Vignuzzi M (2017) Curcumin inhibits Zika and chikungunya virus infection by inhibiting cell binding. Antivir Res 142:148–157
Rausch K, Hackett BA, Weinbren NL, Reeder SM, Sadovsky Y, Hunter CA, Schultz DC, Coyne CB, Cherry S (2017) Screening bioactives reveals nanchangmycin as a broad spectrum antiviral active against Zika virus. Cell Rep 18(3):804
Röcker AE, Müller JA, Dietzel E, Harms M, Krüger F, Heid C, Sowislok A, Riber CF, Kupke A, Lippold S, von Einem J (2018) The molecular tweezer CLR01 inhibits Ebola and Zika virus infection. Antiviral Res 152:26–35
Oo A, Teoh BT, Sam SS, Bakar SA, Zandi K (2019) Baicalein and baicalin as Zika virus inhibitors. Adv Virol 164(2):585–593
Gao Y, Tai W, Wang N, Li X, Jiang S, Debnath AK, Du L, Chen S (2019) Identification of novel natural products as effective and broad-spectrum anti-Zika virus inhibitors. Viruses 11(11):1019
Telehany SM, Humby MS, McGee TD Jr, Riley SP, Jacobs A, Rizzo RC (2020) Identification of Zika virus inhibitors using homology modeling and similarity-based screening to target glycoprotein E. Biochemistry 59(39):3709–3724
Pitts J, Hsia CY, Lian W, Wang J, Pfeil MP, Kwiatkowski N, Li Z, Jang J, Gray NS, Yang PL (2019) Identification of small molecule inhibitors targeting the Zika virus envelope protein. Antivir Res 164:147–153
Sharma N, Prosser O, Kumar P, Tuplin A, Giri R (2020) Small molecule inhibitors possibly targeting the rearrangement of Zika virus envelope protein. Antivir Res 182:104876
Zou M, Liu H, Li J, Yao X, Chen Y, Ke C, Liu S (2020) Structure-activity relationship of flavonoid bifunctional inhibitors against Zika virus infection. Biochem Pharmacol 177:113962
Yu Y, Deng YQ, Zou P, Wang Q, Dai Y, Yu F, Du L, Zhang NN, Tian M, Hao JN, Meng Y (2017) A peptide-based viral inactivator inhibits Zika virus infection in pregnant mice and fetuses. Nat Commun 8(1):1–2
Chen L, Liu Y, Wang S, Sun J, Wang P, Xin Q, Zhang L, Xiao G, Wang W (2017) Antiviral activity of peptide inhibitors derived from the protein E stem against Japanese encephalitis and Zika viruses. Antivir Res 141:140–149
Wang D, Chen C, Liu S, Zhou H, Yang K, Zhao Q, Ji X, Chen C, Xie W, Wang Z, Mi LZ (2017) A mutation identified in neonatal microcephaly destabilizes Zika virus NS1 assembly in vitro. Sci Rep 7(1):1
Raza S, Abbas G, Azam SS (2019) Screening pipeline for Flavivirus based inhibitors for Zika virus NS1. IEEE/ACM Trans Comput Biol Bioinform 17(5):1751–1761
Ahmad N, Badshah SL, Junaid M, Ur Rehman A, Muhammad A, Khan K (2021) Structural insights into the Zika virus NS1 protein inhibition using a computational approach. J Biomol Struct Dyn 39(8):3004–3011
Erbel P, Schiering N, D’Arcy A, Renatus M, Kroemer M, Lim SP, Yin Z, Keller TH, Vasudevan SG, Hommel U (2006) Structural basis for the activation of flaviviral NS3 proteases from dengue and West Nile virus. Nat Struct Mol Biol 13(4):372–373
Lei J, Hansen G, Nitsche C, Klein CD, Zhang L, Hilgenfeld R (2016) Crystal structure of Zika virus NS2B-NS3 protease in complex with a boronate inhibitor. Science 353(6298):503–505
Yuan S, Chan JF, den Haan H, Chik KK, Zhang AJ, Chan CC, Poon VK, Yip CC, Mak WW, Zhu Z, Zou Z (2017) Structure-based discovery of clinically approved drugs as Zika virus NS2B-NS3 protease inhibitors that potently inhibit Zika virus infection in vitro and in vivo. Antivir Res 145:33–43
Roy A, Lim L, Srivastava S, Lu Y, Song J (2017) Solution conformations of Zika NS2B-NS3pro and its inhibition by natural products from edible plants. PLoS ONE 12(7):e0180632
Li Z, Brecher M, Deng YQ, Zhang J, Sakamuru S, Liu B, Huang R, Koetzner CA, Allen CA, Jones SA, Chen H (2017) Existing drugs as broad-spectrum and potent inhibitors for Zika virus by targeting NS2B-NS3 interaction. Cell Res 27(8):1046–1064
Lee H, Ren J, Nocadello S, Rice AJ, Ojeda I, Light S, Minasov G, Vargas J, Nagarathnam D, Anderson WF, Johnson ME (2017) Identification of novel small molecule inhibitors against NS2B/NS3 serine protease from Zika virus. Antivir Res 139:49–58
Lim HJ, Nguyen TT, Kim NM, Park JS, Jang TS, Kim D (2017) Inhibitory effect of flavonoids against NS2B-NS3 protease of ZIKA virus and their structure activity relationship. Biotechnol Lett 39(3):415–421
Chan JF, Chik KK, Yuan S, Yip CC, Zhu Z, Tee KM, Tsang JO, Chan CC, Poon VK, Lu G, Zhang AJ (2017) Novel antiviral activity and mechanism of bromocriptine as a Zika virus NS2B-NS3 protease inhibitor. Antivir Res 141:29–37
Li Y, Zhang Z, Phoo WW, Loh YR, Li R, Yang HY, Jansson AE, Hill J, Keller TH, Nacro K, Luo D (2018) Structural insights into the inhibition of Zika virus NS2B-NS3 protease by a small-molecule inhibitor. Structure 26(4):555–564
Kumar A, Liang B, Aarthy M, Singh SK, Garg N, Mysorekar IU, Giri R (2018) Hydroxychloroquine inhibits Zika virus NS2B-NS3 protease. ACS Omega 3(12):18132–18141
Coronado MA, Eberle RJ, Bleffert N, Feuerstein S, Olivier DS, de Moraes FR, Willbold D, Arni RK (2018) Zika virus NS2B/NS3 proteinase: a new target for an old drug-Suramin a lead compound for NS2B/NS3 proteinase inhibition. Antivir Res 160:118–125
Li Z, Sakamuru S, Huang R, Brecher M, Koetzner CA, Zhang J, Chen H, Qin CF, Zhang QY, Zhou J, Kramer LD (2018) Erythrosin B is a potent and broad-spectrum orthosteric inhibitor of the flavivirus NS2B-NS3 protease. Antivir Res 150:217–225
Santos FR, Nunes DA, Lima WG, Davyt D, Santos LL, Taranto AG, Ferreira MSJ (2019) Identification of Zika virus NS2B-NS3 protease inhibitors by structure-based virtual screening and drug repurposing approaches. J Chem Inf Model 60(2):731–737
Yao Y, Huo T, Lin YL, Nie S, Wu F, Hua Y, Wu J, Kneubehl AR, Vogt MB, Rico-Hesse R, Song Y (2019) Discovery, X-ray crystallography and antiviral activity of allosteric inhibitors of flavivirus NS2B-NS3 protease. J Am Chem Soc 141(17):6832–6836
Rassias G, Zogali V, Swarbrick CM, Chan KW, Chan SA, Gwee CP, Wang S, Kaplanai E, Canko A, Kiousis D, Lescar J (2019) Cell-active carbazole derivatives as inhibitors of the Zika virus protease. Eur J Med Chem 180:536–545
Pathak N, Kuo YP, Chang TY, Huang CT, Hung HC, Hsu JT, Yu GY, Yang JM (2020) Zika virus NS3 protease pharmacophore anchor model and drug discovery. Sci Rep 10(1):1–7
Cui X, Zhou R, Huang C, Zhang R, Wang J, Zhang Y, Ding J, Li X, Zhou J, Cen S (2020) Identification of theaflavin-3, 3’-digallate as a novel Zika virus protease inhibitor. Front Pharmacol. https://doi.org/10.3389/fphar.2020.514313
Pach S, Sarter TM, Yousef R, Schaller D, Bergemann S, Arkona C, Rademann J, Nitsche C, Wolber G (2020) Catching a moving target: comparative modeling of flaviviral NS2B-NS3 reveals small molecule Zika protease inhibitors. ACS Med Chem Lett 11(4):514–520
Voss S, Nitsche C (2020) Inhibitors of the Zika virus protease NS2B-NS3. Bioorg Med Chem Lett 30(5):126965
Akaberi D, Chinthakindi PK, Båhlström A, Palanisamy N, Sandström A, Lundkvist Å, Lennerstrand J (2020) Identification of a C2-symmetric diol based human immunodeficiency virus protease inhibitor targeting Zika virus NS2B-NS3 protease. J Biomol Struct Dyn 38(18):5526–5536
Coluccia A, Puxeddu M, Nalli M, Wei CK, Wu YH, Mastrangelo E, Elamin T, Tarantino D, Bugert JJ, Schreiner B, Nolte J (2020) Discovery of Zika virus NS2B/NS3 inhibitors that prevent mice from life-threatening infection and brain damage. ACS Med Chem Lett 11(10):1869–1874
Lima CS, Mottin M, de Assis LR, Mesquita NC, de Paula Sousa BK, Coimbra LD, Bispo-dos-Santos K, Zorn KM, Guido RV, Ekins S, Marques RE (2021) Flavonoids from Pterogyne nitens as Zika virus NS2B-NS3 protease inhibitors. Bioorg Chem 109:104719
Nie S, Yao Y, Wu F, Wu X, Zhao J, Hua Y, Wu J, Huo T, Lin YL, Kneubehl AR, Vogt MB (2021) Synthesis, structure–activity relationships, and antiviral activity of allosteric inhibitors of Flavivirus NS2B–NS;3 protease. J Med Chem 64(5):2777–2800
Nie S, Zhao J, Wu X, Yao Y, Wu F, Lin YL, Li X, Kneubehl AR, Vogt MB, Rico-Hesse R, Song Y (2021) Synthesis, structure-activity relationship and antiviral activity of indole-containing inhibitors of Flavivirus NS2B-NS3 protease. Eur J Med Chem 225:113767
Chong Teoh T, Al-Harbi JS, Abdulrahman AY, Rothan HA (2021) Doxycycline interferes with Zika virus serine protease and inhibits virus replication in human skin fibroblasts. Molecules 26(14):4321
Shiryaev SA, Farhy C, Pinto A, Huang CT, Simonetti N, Ngono AE, Dewing A, Shresta S, Pinkerton AB, Cieplak P, Strongin AY (2017) Characterization of the Zika virus two-component NS2B-NS3 protease and structure-assisted identification of allosteric small-molecule antagonists. Antivir Res 143:218–229
Nitsche C, Zhang L, Weigel LF, Schilz J, Graf D, Bartenschlager R, Hilgenfeld R, Klein CD (2017) Peptide–boronic acid inhibitors of flaviviral proteases: medicinal chemistry and structural biology. J Med Chem 60(1):511–516
Jackman JA, Costa VV, Park S, Real AL, Park JH, Cardozo PL, Ferhan AR, Olmo IG, Moreira TP, Bambirra JL, Queiroz VF (2018) Therapeutic treatment of Zika virus infection using a brain-penetrating antiviral peptide. Nat Mater 17(11):971–977
Phoo WW, Zhang Z, Wirawan M, Chew EJ, Chew AB, Kouretova J, Steinmetzer T, Luo D (2018) Structures of Zika virus NS2B-NS3 protease in complex with peptidomimetic inhibitors. Antivir Res 160:17–24
Nitsche C, Passioura T, Varava P, Mahawaththa MC, Leuthold MM, Klein CD, Suga H, Otting G (2019) De novo discovery of nonstandard macrocyclic peptides as noncompetitive inhibitors of the Zika virus NS2B-NS3 protease. ACS Med Chem Lett 10(2):168–174
Abdulrahman AY, Khazali AS, Teoh TC, Rothan HA, Yusof R (2019) Novel peptides inhibit zika NS2B-NS3 serine protease and virus replication in human hepatic cell line. Int J Pept Res Ther 25(4):1659–1668
Sahoo M, Lingaraja Jena SD, Kumar S (2016) Virtual screening for potential inhibitors of NS3 protein of Zika virus. Genom Inform 14(3):104
Byler KG, Ogungbe IV, Setzer WN (2016) In-silico screening for anti-Zika virus phytochemicals. J Mol Graph Model 69:78–91
Fatima M, Khan MS, Rashid H, Mehmood A, Kanwal S, Rasheed MA, Jamil F (2018) Structure based virtual screening and molecular docking studies for identification of allosteric inhibitors against Zika virus protease NS2B-NS3. Pak J Zool 50(5):1709–1715
Rohini K, Agarwal P, Preethi B, Shanthi V, Ramanathan K (2019) Exploring the lead compounds for Zika virus NS2B-NS3 protein: an e-pharmacophore-based approach. Appl Biochem Biotechnol 187(1):194–210
Bowen LR, Li DJ, Nola DT, Anderson MO, Heying M, Groves AT, Eagon S (2019) Identification of potential Zika virus NS2B-NS3 protease inhibitors via docking, molecular dynamics and consensus scoring-based virtual screening. J Mol Model 25(7):1
Choudhry H, Alzahrani FA, Hassan MA, Alghamdi A, Abdulaal WH, Bakhrebah MA, Zamzami MA, Helmi N, Bokhari FF, Zeyadi M, Baothman OA (2019) Zika virus targeting by screening inhibitors against NS2B/NS3 protease. Biomed Res Int. https://doi.org/10.1155/2019/3947245
Thirumoorthy GS, Tarachand SP, Nagella P, Veerappa Lakshmaiah V (2021) Identification of potential ZIKV NS2B-NS3 protease inhibitors from Andrographis paniculata: an in-silico approach. J Biomol Struct Dyn. https://doi.org/10.1080/07391102.2021.1956592
Shin HJ, Kim MH, Lee JY, Hwang I, Yoon GY, Kim HS, Kwon YC, Ahn DG, Kim KD, Kim BT, Kim SJ (2021) Structure-based virtual screening: identification of a novel NS2B-NS3 protease inhibitor with potent antiviral activity against Zika and Dengue viruses. Microorganisms 9(3):545
Zhao B, Yi G, Du F, Chuang YC, Vaughan RC, Sankaran B, Kao CC, Li P (2017) Structure and function of the Zika virus full-length NS5 protein. Nat Commun 8(1):1–9
Zmurko J, Marques RE, Schols D, Verbeken E, Kaptein SJ, Neyts J (2016) The viral polymerase inhibitor 7-deaza-2’-C-methyladenosine is a potent inhibitor of in vitro Zika virus replication and delays disease progression in a robust mouse infection model. PLoS Negl Trop Dis 10(5):e0004695
Xu HT, Hassounah SA, Colby-Germinario SP, Oliveira M, Fogarty C, Quan Y, Han Y, Golubkov O, Ibanescu I, Brenner B, Stranix BR (2017) Purification of Zika virus RNA-dependent RNA polymerase and its use to identify small-molecule Zika inhibitors. J Antimicrob Chemother 72(3):727–734
Bullard-Feibelman KM, Govero J, Zhu Z, Salazar V, Veselinovic M, Diamond MS, Geiss BJ (2017) The FDA-approved drug sofosbuvir inhibits Zika virus infection. Antivir Res 137:134–140
Wang C, Yang SN, Smith K, Forwood JK, Jans DA (2017) Nuclear import inhibitor N-(4-hydroxyphenyl) retinamide targets Zika virus (ZIKV) nonstructural protein 5 to inhibit ZIKV infection. Biochem Biophys Res Commun 493(4):1555–1559
Lu G, Bluemling GR, Collop P, Hager M, Kuiper D, Gurale BP, Painter GR, De La Rosa A, Kolykhalov AA (2017) Analysis of ribonucleotide 5′-triphosphate analogs as potential inhibitors of Zika virus RNA-dependent RNA polymerase by using nonradioactive polymerase assays. Antimicrob Agents Chemother 61(3):e01967-e2016
Tong X, Smith J, Bukreyeva N, Koma T, Manning JT, Kalkeri R, Kwong AD, Paessler S (2018) Merimepodib, an IMPDH inhibitor, suppresses replication of Zika virus and other emerging viral pathogens. Antivir Res 149:34–40
Yang S, Xu M, Lee EM, Gorshkov K, Shiryaev SA, He S, Sun W, Cheng YS, Hu X, Tharappel AM, Lu B (2018) Emetine inhibits Zika and Ebola virus infections through two molecular mechanisms: inhibiting viral replication and decreasing viral entry. Cell Discov 4(1):1–4
Pattnaik A, Palermo N, Sahoo BR, Yuan Z, Hu D, Annamalai AS, Vu HL, Correas I, Prathipati PK, Destache CJ, Li Q (2018) Discovery of a non-nucleoside RNA polymerase inhibitor for blocking Zika virus replication through in silico screening. Antivir Res 151:78–86
Lin Y, Zhang H, Song W, Si S, Han Y, Jiang J (2019) Identification and characterization of Zika virus NS5 RNA-dependent RNA polymerase inhibitors. Int J Antimicrob Agents 54(4):502–506
Gharbi-Ayachi A, Santhanakrishnan S, Wong YH, Chan KW, Tan ST, Bates RW, Vasudevan SG, El Sahili A, Lescar J (2020) Non-nucleoside inhibitors of Zika virus RNA-dependent RNA polymerase. J Virol 94(21):e00794-e820
Yuan J, Yu J, Huang Y, He Z, Luo J, Wu Y, Zheng Y, Wu J, Zhu X, Wang H, Li M (2020) Antibiotic fidaxomicin is an RdRp inhibitor as a potential new therapeutic agent against Zika virus. BMC Med 18(1):1–6
Chen Y, Li Z, Pan P, Lao Z, Xu J, Li Z, Zhan S, Liu X, Wu Y, Wang W, Li G (2021) Cinnamic acid inhibits Zika virus by inhibiting RdRp activity. Antivir Res 192:105117
Sáez-Álvarez Y, Jiménez de Oya N, Del Águila C, Saiz JC, Arias A, Agudo R, Martín-Acebes MA (2021) Novel non-nucleoside inhibitors of Zika virus polymerase identified through the screening of an open library of anti-kynetoplastid compounds. Antimicrob Agents Chemother. https://doi.org/10.1128/AAC.00894-21
Stephen P, Baz M, Boivin G, Lin SX (2016) Structural insight into NS5 of Zika virus leading to the discovery of MTase inhibitors. J Am Chem Soc 138(50):16212–16215
Hercik K, Brynda J, Nencka R, Boura E (2017) Structural basis of Zika virus methyltransferase inhibition by sinefungin. Adv Virol 162(7):2091–2096
Hernandez J, Hoffer L, Coutard B, Querat G, Roche P, Morelli X, Decroly E, Barral K (2019) Optimization of a fragment linking hit toward Dengue and Zika virus NS5 methyltransferases inhibitors. Eur J Med Chem 161:323–333
Spizzichino S, Mattedi G, Lauder K, Valle C, Aouadi W, Canard B, Decroly E, Kaptein SJ, Neyts J, Graham C, Sule Z (2020) Design, synthesis and discovery of N, N’-carbazoyl-aryl-urea Inhibitors of Zika NS5 methyltransferase and virus replication. ChemMedChem 15(4):385
Song W, Zhang H, Zhang Y, Chen Y, Lin Y, Han Y, Jiang J (2021) Identification and characterization of Zika Virus NS5 methyltransferase inhibitors. Front Cell Infect Microbiol 11:267
Elfiky AA (2016) Zika viral polymerase inhibition using anti-HCV drugs both in market and under clinical trials. J Med Virol 88(12):2044–2051
Singh A, Jana NK (2017) Discovery of potential Zika virus RNA polymerase inhibitors by docking-based virtual screening. Comput Biol Chem 71:144–151
Ramharack P, Soliman ME (2018) Zika virus NS5 protein potential inhibitors: an enhanced in silico approach in drug discovery. J Biomol Struct Dyn 36(5):1118–1133
Elfiky AA (2020) Novel guanosine derivatives against Zika virus polymerase in silico. J Med Virol 92(1):11–16
Ali R, Badshah SL, Faheem M, Abbasi SW, Ullah R, Bari A, Jamal SB, Mahmood HM, Haider A, Haider S (2020) Identification of potential inhibitors of Zika virus NS5 RNA-dependent RNA polymerase through virtual screening and molecular dynamic simulations. Saudi Pharm J 28(12):1580–1591
Zhang C, Feng T, Cheng J, Li Y, Yin X, Zeng W, Jin X, Li Y, Guo F, Jin T (2017) Structure of the NS5 methyltransferase from Zika virus and implications in inhibitor design. Biochem Biophys Res Commun 492(4):624–630
Santos FR, Lima WG, Maia EH, Assis LC, Davyt D, Taranto AG, Ferreira JM (2020) Identification of a potential Zika virus inhibitor targeting NS5 methyltransferase using virtual screening and molecular dynamics simulations. J Chem Inf Model 60(2):562–568
Bharadwaj S, Rao AK, Dwivedi VD, Mishra SK, Yadava U (2021) Structure-based screening and validation of bioactive compounds as Zika virus methyltransferase (MTase) inhibitors through first-principle density functional theory, classical molecular simulation and QM/MM affinity estimation. J Biomol Struct Dyn 39(7):2338–2351
Kingwell K (2021) Pan-serotype antiviral for dengue infection. Nat Rev Microbiol 19(4):221
Scroggs SL, Gass JT, Chinnasamy R, Widen SG, Azar SR, Rossi SL, Arterburn JB, Vasilakis N, Hanley KA (2021) Evolution of resistance to fluoroquinolones by dengue virus serotype 4 provides insight into mechanism of action and consequences for viral fitness. Virology 552:94–106
Acknowledgements
Shobana Sundar thanks the Indian Council of Medical Research (ICMR) for the financial support through the award of Research Associateship (ISRM/11(13)/2019). The authors also thank the Department of Bioinformatics, Bharathiar University for the computational facilities.
Author information
Authors and Affiliations
Contributions
Conceptualization: SS, SP, JN; Writing—original draft preparation: SS; Writing—review and editing: SP, JN; Supervision: SP, JN.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Informed consent
As the study does not involve any human participants and/or Animals, consent from the participants is not required for this study.
Research involving human participants and/or animals statement
The authors declare that the study does not involve any human participants and/or Animals.
Additional information
Edited by Detlev H. Kruger.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sundar, S., Piramanayagam, S. & Natarajan, J. A review on structural genomics approach applied for drug discovery against three vector-borne viral diseases: Dengue, Chikungunya and Zika. Virus Genes 58, 151–171 (2022). https://doi.org/10.1007/s11262-022-01898-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11262-022-01898-5